Heavy Water
Heavy water is deuterium oxide (D2O)
Preparation
1) By prolonged electrolysis
When ordinary water is electrolysed, protium is liberated much more readily than deuterium because of the following reason:
a) Being smaller in size, H+ ions have greater mobility as compared to D+ ions.
b) Because of lower discharge potential, H+ ions are discharged at the cathode more easily than D+ions.
c)Hydrogen atoms combine much more readily to form molecular hydrogen than do deuterium atoms to form D2.
As the electrolysis continues ,the concentration of heavy water in ordinary water gradually increases.If electrolysis is continued till only a small volume remains, then almost pure heavy water is obtained.
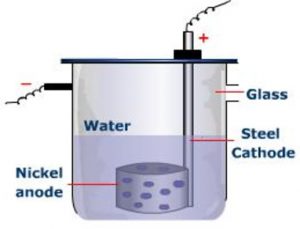
The electrolytic cell designed by Brown, Degget and Urey for the preparation of heavy water is:
1) It consist of a steel cell 45 cm long and 10 cm in diameter which acts as a cathode.
2) The anode is a cylindrical sheet of nickel with a number of holes a large number of holes punched in it.
3) A large number of such cells are employed for electrolysis of water in number of stages.
2) By fractional distillation
At normal atmospheric pressure the boiling point of ordinary and heavy water are 373 K and 374.42 K. Small difference in their boiling points forms the basis of preparation of heavy water by fractional distillation of ordinary water. Fractionating column of the order of about 12 metres height are employed.
Physical properties of heavy water
1)It is colourless, odourless and tasteless mobile liquid heavier than water. Because of higher molecular mass there is a marked difference in physical properties of ordinary water and heavy water.
2)Dielectric constant of heavy water is lower than that of ordinary water therefore ionic compounds are less soluble in heavy water than in ordinary water.
Chemical properties of heavy water
The reactions of heavy water with most of the substances proceed at a much slower rate as compared to ordinary water.
1) Electrolysis : When heavy water is electrolysed, dideuterium is obtained at the cathode.
2D2O ——-> 2D2 + O2
2) Action of alkali and alkaline earth metal
Heavy water reacts slowly with alkali and alkaline earth metals producing dideuterium.
2Na + 2D2O ——> 2NaOD + D2
Ca + 2 D2O ——>Ca (OD)2 + D2
3) Action of metallic oxides
The oxides of active metals like Sodium and Calcium reacts slowly with heavy water to form their respective deuteroxides.
Na2O + D2O ——-> 2NaOD
CaO + D2O —-> Ca(OD)2
4) Action of non -metallic oxides
Non-metallic oxides such as Phosphorus pentoxide ,sulphur trioxide readily dissolved in heavy water forming their corresponding deuteroacids.
P2O5 + 3D2O ——–> 2 D3PO4 (Deuterophosphoric acid)
SO3 + D2O ——–> D2SO4 (Deuterosulphuric acid)
5) Action of metallic carbides
Heavy water reacts with metallic carbides forming deuterohydrocarbons.
Al4C3 + 12 D2O ——> 4 Al(OD)3 + 3 CD4
CaC2 + 2 D2O —-> Ca(OD)2 + DC≡CD
6) Action with metallic nitrides, phosphides and arsenides
Heavy water react with metallic nitrides ,phosphide and nitrides liberating deuteroammonia, deuterophosphine , deuteroarsine.
Mg3N2 + 6 D2O ——>3 Mg (OD)2 + 2ND3
Ca3P2 + 6D2O ——-> 3Ca(OD)2 + 2PD3
Na3As + 3 D2O ——-> 3NaOD + ASD3
7) Formation of deuterates
Heavy water also combined with many compounds as heavy water of crystallization.
For ex: CuSO4·5D2O , Na2SO4 ·10 D2O , MgSO4 ·7 D2O
The heavy hydrates thus obtained are called deuterates.
8) Exchange reaction
When treated with heavy water ,many compounds exchange their active hydrogen atoms either partially or completely with deuterium.
HCl + D2O DCl + HOD
NaOH + D2O NaOD + HOD
CHCl3 + D2O CDCl3 + HOD
NH3DCl + D2O NH2D2Cl + HOD
NH2D2Cl + D2O NHD3Cl + HOD
NHD3Cl + D2O ND4Cl + HOD
9) Biological properties
Heavy water is injurious to human beings, plants and animals since it slows down the rates of reaction occurring in them.
Uses of heavy water
1) As a moderator:
It is extensively used as a moderator in nuclear reactions since it slows down the fast moving neutrons and thus helps in controlling the nuclear reactions.
2) As a tracer compound:
Heavy water is widely used as a tracer compound for studying the mechanism of many reactions.
3) For preparation of dideuterium
Heavy hydrogen or dideuterium is produced by the electrolysis of heavy water or by its decomposition with sodium metal.
Very nice post, great website
Thanks madhav
Helpful notes….
Keep it up mam
It is really helpful