If sufficient energy is supplied, electrons may be removed resulting in the formation of a positively charged ion.
The minimum amount of energy required to remove the most loosely bound electron from an isolated gaseous atom so to convert it into gaseous cation is called ionisation enthalpy.
It is represented by Δ i H
This process may be represented as
M (g) + Δ i H ———–> M + (g) + e – (g)
where M (g) and M + (g) represent the gaseous atom and the resultant gaseous cation.
Ionisation enthalpy is also known as ionisation potential since it is the minimum potential difference required to remove the most loosely bound electrons from an isolated gaseous cation.
It is measured in units of electron volts (eV) per atom or kilo calorie per mole or kilo joules per mole.
One electron volt is the energy acquired by an electron while moving under a potential difference of 1 volt.
The energy required to remove the most loosely bound electrons from the isolated gaseous atom is called its first ionisation enthalpy and is denoted by Δ i H 1
M (g) + Δ i H 1 ———–> M + (g) + e – (g)
The energies required to knock out second and third electrons are called second and third ionisation energies.
M + (g) + Δ i H 2 ———–> M 2+ (g) + e – (g)
M 2+ (g) + Δ i H 3 ———–> M 3+ (g) + e – (g)
When one electron has been removed from the neutral gaseous atom the positively charged ions formed has 1 electrons less than the number of protons in the nucleus. As a result the electrostatic attraction between the nucleus and the remaining electrons in the cation increases i.e. effective nuclear charge increases. The positive ion holds its remaining electrons more firmly. Therefore, the energy required to remove another electron from this positively charged Ion or second electron from the neutral atom must be higher than the first.
The removal of two electrons from the neutral atom give a doubly positively charged ion which will hold its remaining electrons even more tightly. As a result, the energy required to remove third electron from the gaseous atom should be even more than that require for the second electron.
Factors governing the ionization enthalpy
1) Nuclear charge
2) Atomic size
3) Penetration effect of the electrons
4) Screening effect of inner electrons
5) Effect of exactly half filled and completely filled orbitals
Nuclear charge
The ionization enthalpy increases with increase in nuclear charge. With increase in nuclear charge, the electrons of the outer shell are more firmly held by the nucleus and thus greater energy is required to pull out an electron from the atom.
The ionisation enthalpy increases as we move along a period from left to right due to increased nuclear charge.
Atomic Size
Ionisation enthalpy decreases as the atomic size increases. As the distance of the outer electrons from the nucleus increases with increase the atomic radius, the attractive force on the outer electrons decreases. As a result, outer electrons are held less firmly and hence lesser amount of energy is required to knock them out. Ionisation enthalpy decreases with increase in atomic size.
Penetration effect
Ionisation enthalpy increases as the penetration effect of the electrons increases .In case of multi electron atoms, the electrons of the s-orbital has the maximum probability of being found near the nucleus and this probability goes on decreasing in the case of p, d and f orbital of the same shells. s-electrons of any shell are more penetrating towards the nucleus than p- electrons of the same shell. Within the same shell, the penetration effect decreases in the order:
s >p > d> f
If the penetration effect of the electron is more, it will be closer to the nucleus and hence will be held more firmly by the nucleus .Consequently the ionization enthalpy will be high.
Ionisation enthalpy increases with the increase in the penetration effect of electrons.
The ionization enthalpy will be more to knock out a s- electrons than p-electron of the same shell, which in turn, will be more than that required to remove d- electron and so on.
First ionisation enthalpy of Aluminium is lower than that of magnesium
In case of aluminium ( 1s2 2s2 2p6 3s2 3p1 )we have to pull out a p electron to form Al 3+ ion whereas in case of magnesium( 1s2 2s2 2p6 3s2 ) we have to remove an s- electron of the same energy shell to produce Mg + ion. Since the energy required to remove p- electron is lower than that required to knock out s electron of the same energy shell, therefore the first ionization enthalpy of Aluminium is lower than that of the magnesium.
Shielding or Screening Effect of the inner electrons
As the shielding or screening effect of the inner electrons increases, the ionization enthalpy decreases.
In multi electron atoms, the electrons in the valence shell experience an attractive force from the nucleus and a repulsive force from the electrons in the inner shells.The attractive force exerted by the nucleus on the valence shell electrons is somewhat reduced by the repulsive force exerted by the electrons present in the inner shell. The valence shell electrons do not feel the full charge of the nucleus. The actual charge felt by the valence shell electrons is called effective nuclear charge and the repulsive force felt by the valence shell electrons from the electrons present in the inner shell is called the shielding effect or screening effect.
Z eff = Total nuclear charge ( Z) – Screening constant (σ)
Greater the number of electrons in the inner shells, larger will be the screening effect.
As the screening effect increases, the effective nuclear charge decreases.
The force of attraction by the nucleus for the valence shell electrons decreases and hence the ionisation enthalpy decreases.
An increase in the number of electrons in the inner shells tends to decrease the ionization enthalpy.
Electronic configuration
If an atom contains exactly half filled or completely filled orbitals, then such an arrangement has extra stability. Therefore, the removal of an electron from such an atom requires more energy than expected.
1)Be (1s2 2s2 ) has higher ionization enthalpy than B ( 1s2 2s2 2p1 )because Be has fully filled orbitals which is a stable electronic arrangement.
2)Mg(1s2 2s2 2p6 3s2) has higher ionization enthalpy than aluminium ( 1s2 2s2 2p6 3s2 3p1)
3)N ( 1s2 2s2 2px 1 2py 1 2pz 1) has higher ionization enthalpy than oxygen(1s2 2s2 2px 2 2py 1 2pz 1 ) because N contains exactly half filled p orbitals which gives extra stability to the atom.
4)Ionization enthalpy of P (1s2 2s2 2p 6 3 s2 3px 1 3py 1 3pz 1 ) is higher than that of S ( 1s2 2s2 2p 6 3 s2 3p 2 3py1 3pz 1 )
Noble gases have the highest ionization enthalpy in their respective period. Ionization enthalpy of Ne is more than any other element of the second period. This is due to the fact that ns2 np6 arrangement which occurs in noble gases is highly stable and hence larger amount of energy is needed to remove an electron from this stable arrangement.
Variation of ionisation enthalpy
Alkali metals with 1 electron in the outermost s-orbital have lowest ionization enthalpy and hence are highly reactive.
Noble gases with stable ns2 np6 configuration have highest ionization enthalpy and hence are chemically inert.
Variation along a period
As we move from left to right in a period, the ionization enthalpy increases with increasing atomic numbers.
Reason:
As we move across a period from left to right, the nuclear charge increases and the atomic radius decreases though the principal quantum number of the valence shell remains the same.As a result of increased nuclear charge and simultaneous decrease in atomic radius, the valence electrons are more and more tightly held by the nucleus as we move from left to right in a period. More and more energy is needed to remove the electron and hence ionization enthalpy is keep on increasing.
Li to Be :As we move from Li to Be, the ionization enthalpy increases due to increased nuclear charge and smaller atomic radius of Be as compared to that of Li.
Be to B:The ionization enthalpy of B is lower than that of Be. This is due to:
1)The outermost electron in B is present in 2p orbitals while in Be it is present in 2s orbital. Since 2s electrons are more penetrating towards the nucleus than 2p electrons, therefore, lesser amount of energy is required to Knock out a 2p electron than 2s electron. The first ionization enthalpy of Boron is lower than that of Be.
2)The 2p electron of B is not strongly attracted by the nucleus as the 2s electron of beryllium. Consequently the first ionization enthalpy of Boron is lower than that of beryllium.
B to C to N :As we move from Boron to carbon to nitrogen, the first ionization enthalpy of these elements keeps on increasing due to progressively increasing nuclear charge and decreasing atomic radius.
N to O :The first ionization enthalpy of oxygen is lower than that of Nitrogen although the nuclear charge of oxygen is higher than that of Nitrogen.
1)The electronic configuration of N in which the 2p- orbitals are exactly half filled is more stable than the electronic configuration of oxygen in which the 2p orbitals are neither half and nor completely filled. It is difficult to remove an electron from N than from O. The first ionization enthalpy of nitrogen is higher than that of oxygen.
2)The removal of an electron from oxygen gives a stable electronic configuration with exactly half filled 2p subshell while this is not so in case of N. The removal of an electron from oxygen give the most stable electronic configuration than that obtainable from nitrogen.
O to F to Ne: First ionization enthalpy increases from oxygen to fluorine to neon because of the increasing nuclear charge. Neon, the noble gas, has the highest first ionization enthalpy amongst the elements of the 2nd period because of its stable electronic configuration.
Variations within a group
Ionisation enthalpy keep on decreasing regularly as we move down a group from one element to the other.
On moving down the group, the atomic size increases gradually due to the addition of 1 new principal energy shell at succeeding element. As a result, the distance of the valence electron from the nucleus increases. The force of attraction by the nucleus for the valence electron decreases and hence the ionization enthalpy should decrease.
1)With the addition of new shells, the number of inner electron shells which shield the valence electron from the nucleus increases. As a result the force of attraction of nucleus for the valence electrons further decreases and hence the ionization enthalpy decreases.
2)Nuclear charge increases with increase in atomic number. As a result, the force of attraction by the nucleus for the valence electrons should increase and accordingly the ionization enthalpy should decrease.
3)The valence electrons become less and less firmly held by the nucleus and hence the ionization enthalpy is gradually decreases as we move down the group.
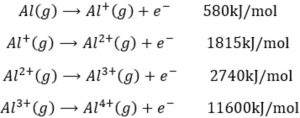

helpful maa’m
LIKE THIS
LAST MOMENTS
Very very nice post .Thank u for sharing this wonderful knowledge
Thanks mam, it’s awesome and very helpful…☺☺
The subshell configuration of Aluminium is incorrect. According to the configuration given in the article, there are only 10 electrons represented in the configuration. ”2p^6” is missing from the configuration. It should be ”1s^2,2s^2,2p^6,3s^2,3p^1”.
I have made the correction, Thanks! Sharvari
This is really, really helpful! However I think it would be important to add the fact about the order of screening effect
i.e. s>p>d>f
Overall , the answer is very well written
I did not understand how Al became Al3+ and Mg became Mg2+
Very good information
Thank you madam.
Thanks it is really helpful
Very very helpfull….
nice notes and helpful
Helpful because I can’t understand only ionisation enthalpy in this chapter but due to this notes I felt so much relaxed. Thanks mam
Great, it helped me alot .
Keep it up
Thanku mam ….. These are very helpful
Thanku mam……..it is really helpful
This is very helpful mam..Thank you so much.
Tomorrow is my chemistry exam…And I want to achieve good marks…
wonderful notes but if it would’ve been a little bit shorter it would’ve been convenient to read as it would be saving a lot of time….