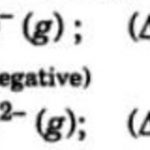Periodicity of valence or Oxidation State The electrons present in the outermost shell of an atom are called valence electrons and the number of these electrons determine the valence or the valency of the atom. The orbitals present in the valence shell are called valence orbitals. The valence of an atom equal to either the number of valence electrons are equal to 8 … [Read more...] about Periodic Trends In Chemical Properties
Classification of Elements and Periodicity in Properties
Electronegativity
Electronegativity of an element is the tendency of its atoms to attract the shared pair of electrons towards itself in a covalent bond. The electronegativity of any given element depends upon the following factors: 1) State of hydridization A sp-hybridized carbon is more electronegative than sp2 hybridised carbon, which in turn is more electronegative than a sp3 … [Read more...] about Electronegativity
Electron Gain Enthalpy
Electron gain Enthalpy Electron gain enthalpy of an element may be defined as the energy released when a neutral isolated gaseous atom accepts an extra electron to form the gaseous negative Ion i.e. anion. It is denoted by Δ eg H. Greater the amount of energy released in the above process, higher is the electron gain enthalpy of the element. The electron gain enthalpy … [Read more...] about Electron Gain Enthalpy
Atomic Radius
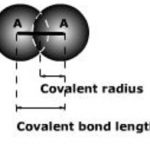
Properties of the individual atoms Properties like valency ,atomic and ionic radii, ionisation enthalpy ,electron gain enthalpy, electronegativity are the properties of the individual atoms and are directly related to the electronic configuration. Properties of group of atoms Properties like melting point, boiling point ,heat of fusion and vaporisation, energy of … [Read more...] about Atomic Radius
Prediction of period, group and block of a given element
The period of an element corresponds to the principal quantum number of the valence shell. The block of an element corresponds to the type of orbital which receive the last electron. The group of an element is predicted from the number of electrons in the valence shell or/and penultimate shell as follows: a)For s block elements ,group number is equal to the number of … [Read more...] about Prediction of period, group and block of a given element