Properties of the individual atoms
Properties like valency ,atomic and ionic radii, ionisation enthalpy ,electron gain enthalpy, electronegativity are the properties of the individual atoms and are directly related to the electronic configuration.
Properties of group of atoms
Properties like melting point, boiling point ,heat of fusion and vaporisation, energy of atomisation, density ,atomic volume are the bulk properties i.e. the properties of a collection or a group of atoms and are only indirectly related to the electronic configuration.
All these properties which are directly or indirectly related to the atomic structure or the electronic configuration of the elements are called atomic properties. Since the electronic configuration of the elements are a periodic function of their atomic numbers therefore these atomic properties are also a periodic function of atomic number of the elements.
The properties which are directly or indirectly related to their electronic configuration and which show a regular gradation when we move from left to right in a period or from top to bottom in a group are called periodic properties.
Atomic radius
The distance from the centre of the nucleus to the outermost shell containing electrons.
or
The distance from the centre of the nucleus to the point up to which the density of the electron cloud is maximum.
Types of atomic radii
1) Covalent radius
2) Van der waals radius
3) Metallic radius
Covalent radius
It is defined as one half the distance between the nuclei of two covalently bonded atoms of the same element in a molecule.
r covalent = ½ (internuclear distance between two bonded atoms)
Since the inter nuclear distance between two bonded atoms is called the bond length. Therefore,
r covalent = ½( bond length)
Van der waals radius
It Is defined as one half the distance between the nuclei of two identical non bonded isolated atoms or two adjacent identical atoms belonging to two neighbouring molecules of an element in the solid state.
The magnitude of the van der waal radius depends upon the packing of the atoms when the element is in the solid state.
For Example: The inter nuclear distance between two adjacent chlorine atoms of the two neighbouring molecules in the solid state is 360 pm. The van der waals radius of chlorine atom is 180 pm.
The inter nuclear distance between two adjacent hydrogen atoms of the two neighbouring molecules in the solid state 240 pm .The van der waals radius is 120 pm.
Van der waals radius of an element is always larger than its covalent radius because:
1)Since the van der waal forces of attraction are weak ,therefore, the internuclear distance in case of atoms held by van der waal forces are much larger than those between covalently bonded atoms.
2)Since a covalent bond is formed by overlap of two half filled atomic orbitals, a part of electron cloud become common. Therefore covalent radii are always smaller than van der waal radius.
Metallic radius
A metal lattice or crystal consists of positive kernels or metal ions arranged in a definite pattern in a sea of mobile valence electrons. Each kernel is simultaneously attracted by a number of mobile electrons and each mobile electron is attracted by a number of metal ions. Force of attraction between the mobile electrons and the positive kernels is called the metallic bond.
It is defined as one half the internuclear distance between the two adjacent metal ions in the metallic lattice.
In a metallic lattice, the valence electrons are mobile ,therefore, they are only weakly attracted by the metal ions or kernels. In a covalent bond ,a pair of electrons are strongly attracted by the nuclei of two atoms. Thus a metallic radius is always longer than its covalent radius.
Metallic radius of sodium is 186 pm whereas its covalent radius as determined from its vapour which exist as Na2 is 154 pm. Metallic radius of Potassium is 231pm while its covalent radius is 203 pm.
Variation of atomic radii in the periodic table
The covalent and van der waals radii decreases with increase in atomic number as we move from left to right in a period.
The alkali metals which are at the extreme left of the periodic table has the largest size in a period.
The halogens which are present at the extreme right of the periodic table have the smallest size.
The atomic size of nitrogen is the smallest. After nitrogen, atomic size increases for Oxygen and then decreases for fluorine.
In nitrogen ,all the three p orbitals have one electron each. When we move from nitrogen to oxygen, the nuclear charge increases by 1. But at the same time one of the p orbital has now 2 electrons which repel each other.In case of oxygen inter-electronic repulsions overweigh the effect of increased nuclear charge and hence the atomic size increases from nitrogen to oxygen. On moving from O to F, the nuclear charge increases by 1 and at the same time two of the p orbitals now have 2 electrons each which repel each other.The enhanced nuclear charge overweighs the effect of inter electronic repulsion and hence the size decreases from oxygen to fluorine.
The size of atoms of inert gases are larger than those of the preceding halogens.
As we move from left to right in a period, nuclear charge increases by 1 units in each succeeding element while the number of shells remains the same. Due to this enhanced nuclear charge, the electrons of all the shells are pulled little closer to the nucleus thereby making each individual shells smaller and smaller. This result in a decrease of the atomic radius as we move from left to right in a period.
The atomic radius abruptly increases as we move from halogens to the inert gas.
This is due to the reason that in case of inert gases all the orbitals are completely filled and hence the inter-electronic are maximum. The atomic size is expressed in terms of Van der Waals radius since they do not form covalent bonds.
Van der waals radius are larger than covalent radii therefore ,the atomic size of an inert gas in a period is much higher than that of preceding halogen
Variation within a group
The atomic radii of elements increases with increase in atomic number as we move from top to bottom in a group.
As we move down the group the principal quantum Number increases. A new energy shell is added at each succeeding element and the valence electrons lie farther and farther away from the nucleus. As a result the attraction of the nucleus for the electron decreases and hence the atomic radius increases.
With increase in atomic number , the nuclear charge also increases. As a result, the force of attraction of the nucleus for the electrons should increase and hence the atomic radii should decrease. But the effect of increased nuclear charge is reduced due to the screening or shielding effect on the valence electrons by the electrons present in the inner shells. The effect of adding a new energy shell is so large that it overweighs the contractive effect of the increased nuclear charge. Hence the increase in atomic radii as we move down the group is due to addition of new energy shells.
Ionic radii
The ionic radii corresponds to the radii of ions in ionic crystals.
Ionic radius may be defined as the effective distance from the centre of the nucleus of the ion upto which it exerts its influence on its electronic cloud.
The radius of cation is always smaller than that of its parent atom
A cation is formed by loss of one or more electrons from the neutral gaseous atom .This causes the removal of the whole of the outermost shell of electrons.
Due to the removal of the valence shell, the number of shells in the cation decreases. As a result the size of cation is smaller than the parent atom from which it is formed. Due to the removal of electrons from the parent atom, the number of electrons in the cation decreases but its nuclear charge remains the same as that of the atom. The force of attraction by the nucleus on the electron increases and hence the size of the atom decreases.
The radius of the anion is always larger than that of its parent atom
An anion is formed when a neutral gaseous atom gains one or more electrons. This increases the number of electrons in the anion while its nuclear charge remains the same as that on the neutral atom. Since the same nuclear charge now attracts greater number of electrons therefore the force of attraction by the nucleus on the electrons of all the shells decreases. Addition of one or more electrons, increases the repulsions among electrons and the electron cloud of the atom expands.
The ionic radii of anions also increases as we move from top to bottom within a group primary due to an increase in the number of shells.
Isoelectronic ions
Ions of the different elements which have the same number of electrons but different magnitude of the nuclear charge are called isoelectronic ions.
For example : Sulphide ions ( S2-) , Chloride ions (Cl–) and potassium ions ( K +) are isoelectronic ions because each of them has 18 electrons but have different nuclear charge i.e. 16, 17 and 19.
A neutral atom may also have same number of electrons.
For Example : Argon is isoelectronic with S2– , Cl–, K+
Isoelectronic species may be defined as neutral or ionic species which have the same number of electrons but different nuclear charge.
Nitrite ion( N3-) , oxide Ion( O2-) , fluoride Ion (F–), Sodium ion(Na+) and magnesium(Mg2+), iron ( Fe3+), aluminium(Al3+) are all isoelectronic species since each one of them contains 10 electrons but different nuclear charge of 7, 8 ,9 ,10,11, 12 ,13.
As nuclear charge increases, the force of attraction by the nucleus on the electrons also increases. As a result ionic radii decreases.
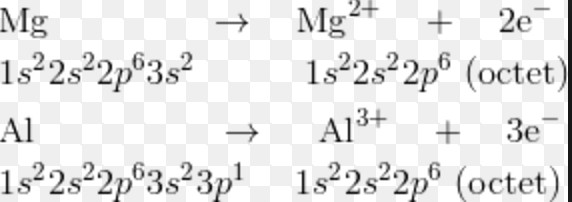
print options ???