In case of certain molecules, a single Lewis structure cannot explain all the properties of the molecule. The molecule is then supposed to have many structures, each of which can explain most of the properties of the molecules but none can explain all the properties of the molecule.
The actual structure is in between of all these constituting structure and is called resonance hybrid and different individual are called resonating structures or Canonical forms.
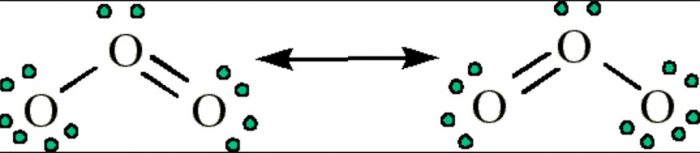
The structure of ozone can be written as
Where each oxygen atom has an octet of electron. But this structure is unsatisfactory because it depicts the central O atom to be bonded to one O atom by a double bond and the other oxygen atom by a single bond. Since double bond is shorter than single bond, the two bond length in this molecule should be unequal.
But experimental evidence not only show that the bond length are equal but also shows that the bonds are intermediate between single and double bond.
For molecules like Ozone, a single Lewis structure is unable to explain the observed facts. Hence an alternative Lewis structure can be written in which the double and single bonds are interchanged.
The actual structure is intermediate between the two Lewis structure and its called resonance hybrid.
Rules for writing of resonating structures
1)The contributing structures should have the same position of atoms. They should differ only in the position of electrons.
2)The contributing structures should have the same number of unpaired electrons.
3)The contributing structure should have nearly same energy.
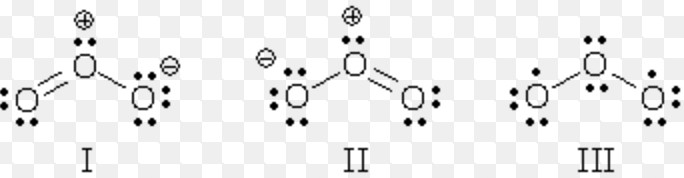
4)The contributing structures should have negative charge on the electronegative atom and the positive charge on the electropositive atoms.
5) In a contributing structure, like charges should not be present on adjacent atom while unlike charges should not be widely separated.
Resonating structure of some molecules
1) Resonating structure of Carbon dioxide
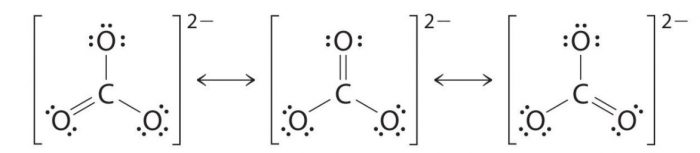
2) Resonating structure of Carbonate ion
3) Resonating structure of Nitrate ion
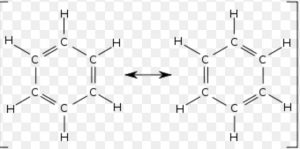
4) Resonating structure of benzene
Resonance Energy
Resonance hybrid is more stable than any of the contributing structure. The resonance hybrid has lower energy than any of the contributing structure.
The difference in the energy of the resonance hybrid and the most stable contributing structure is called resonance energy.
Characteristics of resonance
1) The contributing structures do not have any real existence. They are only imaginary. Only the resonance hybrid has the real existence.
2) As a result of resonance, the bond length in a molecule becomes equal.
3) The resonance hybrid has lower energy and hence greater stability there any of the contributing structure.
4) Greater is the resonance energy, greater is the stability of the molecule.
5) Greater is the number of Canonical forms especially with nearly same energy, greater is the stability of the molecule.
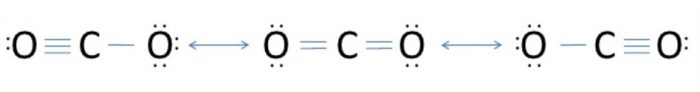
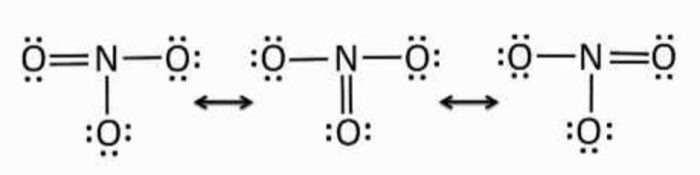
the content is so clear and understandable, pick up
It would be better to explain point no. 2 i.e. ” The contributing structures should have the same number of unpaired electrons” with an example. Is it “unpaired electrons” or “lone pair of electrons”? Which is correct? If unpaired is correct, would appreciate a suitable example and its explanation.
It very easy to understand and very easy to remember…..
It proves very helping.
Very easy language to understand and remember the points.
It is really usefull
It helped me a lot..
Good content ma’am keep it up
Kindly post the notes of hydrocarbons also
It is very useful……and understanding language is used for the students help ……… thanks