Molecular orbital theory
Features of Molecular orbital theory
1) The atomic orbitals overlap to form new orbitals called molecular orbitals.
When two atomic orbitals overlap or combine, they lose their identity and form new orbitals. The new orbitals thus formed are called molecular orbitals.
2) Molecular orbitals are the energy states of a molecule in which the electrons of the molecule are filled just as atomic orbitals are the energy states of an atom in which the electrons of the atoms are filled.
3) A molecular orbitals gives the electron probability distribution around a group of nuclei just as atomic orbitals give the electron probability distribution around the single nucleus.
4) Only those atomic orbitals can combine to form molecular orbitals which have comparable energies and proper orientation.
For example :1s can combine with 1s and not with 2s.
5) The number of molecular orbitals formed is equal to number of combining atomic orbitals.
6) When two atomic orbitals combine, they form two new orbitals called bonding molecular orbitals and antibonding molecular orbitals.
7) The bonding molecular orbitals has lower energy and hence greater stability than the corresponding antibonding molecular orbitals.
8) The bonding molecular orbitals are represented by σ, π, δ whereas the corresponding antibonding molecular orbitals are represented by σ∗, π∗, δ∗
9) The shape of molecular orbitals formed depend upon the type of the combining atomic orbitals.
10) The filling of molecular orbitals takes place according to same rules as those of the atomic orbitals. These are:
1) Aufbau principle : Molecular orbitals are filled in order of the increasing energies.
2) Pauli exclusion principle: Molecular orbital can have maximum of 2 electrons and these must have opposite spin.
3)Hund’s rule of maximum multiplicity :Pairing of electrons in the degenerate molecular orbitals does not take place until each of them has got one electron each.
Formation of molecular orbitals
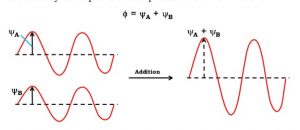
An atomic orbital is an electron wave, the waves of the two atomic orbitals may be in phase or out of phase. Suppose ΨA and ΨB represent the amplitude of the electron wave of the atomic orbitals of the two atoms A and B.
Case 1: When the two waves are in phase so that they add up and amplitude of the wave is
Φ= ΨA + ΨB
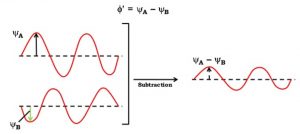
Case 2 : when the two waves are out of phase, the waves are subtracted from each other so that the amplitude of new wave is
Φ ´= ΨA – ΨB
The molecular orbitals formed by the additive effect of the atomic orbitals is called bonding molecular orbitals and the molecular orbitals formed by the subtractive effect of atomic is called antibonding molecular orbitals.
The probability of finding the electrons in the bonding molecular orbital increases where as it decreases in the antibonding molecular orbital.
There is a nodal plane between the two nuclei of an antibonding molecular orbital i.e. a plane on which electron density is zero.
Atomic orbitals are represented by s, p, d, the bonding molecular orbitals are represented by σ, π, δ and the corresponding antibonding molecular orbitals are represented by σ∗, π∗, δ∗.
Crest of the electron wave are usually given by + sign and troughs a – sign.
Bonding molecular orbital is formed by the combination of + and + and – with – part of the electron waves whereas antibonding molecular orbital are formed by the overlap of + with – part.
Compared with the energy of the combining atomic orbitals, the energy of the antibonding molecular orbitals is raised by an amount greater than the amount by which the energy of the bonding molecular orbital is lowered. In the bonding molecular orbital, the electron density in the internuclear region is high. As a result the nuclei are shielded from each other.
Therefore the repulsion between the nuclei are very small. In the antibonding molecular orbital, the electron density in the internuclear region is very low. As a result, the nuclei are directly exposed to each other i.e. there is very less shielding. Hence the repulsion between the nuclei are very large so that antibonding molecular orbital is raised much higher in energy than the bonding molecular orbital is lowered in energy.
The lowering of energy of the bonding molecular than the combining atomic orbital is called stabilization energy.
Increase in energy of the antibonding molecular orbitals is called destabilization energy.
Characteristics of bonding molecular orbitals
1) The probability of finding the electron in the internuclear region of the bonding molecular orbital is greater than that of combining atomic orbitals .
2) The electrons present in the bonding molecular orbital result in the attraction between the two atoms.
3) As a result of attraction, the bonding molecular orbital has lower energy and hence greater stability than that of the combining atomic orbitals.
4) They are formed by the additive effect of the atomic orbitals i.e. crest fall over the crest and troughs over the trough so that the amplitude of the new wave is given by Φ= ΨA + ΨB
5) They are represented by σ, π, δ.
Characteristics of antibonding molecular orbitals
1) The probability of finding the electron in the internuclear region decreases in the antibonding molecular orbitals.
2) The electrons present in the antibonding molecular orbital result in the repulsion between the two atoms.
3) Because of the repulsive force, the antibonding molecular orbitals has higher energy and lower stability i.e. it does not favour the formation of bond.
4)They are formed by the subtractive effect of the atomic orbitals i.e. crests falls over the troughs so that the amplitude of the new wave is given by
Φ ´= ΨA – ΨB
5) They are represented by σ∗, π∗, δ∗.
Conditions for the combination of atomic orbitals
1) The combining atomic orbitals should have comparable energy
For Ex: For homonuclear diatomic molecules, 1s atomic orbital of one atom can combine with 1s atomic orbital of another atom. 2s can combine with 2s, 2p with 2p and so on. 1s cannot combine with 2s, 2s cannot combine with 2p.
2) The combining atomic orbitals must have proper orientation so that they are able to overlap to a considerable extent.
3) The extent of overlapping should be large. Greater the overlap, greater will be the electron density between the nuclei.
Superb
Omg!!!!
I jst luvd d explanation n all d topics were explained in a very easy n detailed manner…
Luvd it…….. Thnx for such a gr8 hlp…
SUPERB EXPLANATION WOW!!!!!!!!!!!!!!
Wonderful explanation…
It’s so easy for teaching for me …..
Very Nice Explanation. Please Launch Your App
First of all its good that it free and notes are simple to understand
Good explanation