Bond Length
When atoms come closer to each other, attraction takes place between them and ,therefore, the potential energy of the system keeps on decreasing till at a particular distance, the potential energy is minimum.
If the atoms are further brought closer ,the repulsion start and therefore, the potential energy of the system begins to increase.
At equilibrium distance the atoms keep on vibrating about their mean position.
The equilibrium distance between the centres of the nuclei of the two bonded atoms is called its Bond length
It is expressed in terms of angstrom or picometer.
It is determined experimentally by x-ray diffraction or electron diffraction method or spectroscopic method.
In an ionic compound ,the bond length is the sum of their ionic radii and in covalent compound, it is the sum of their covalent radii.
For a covalent molecule AB, the bond length is given by d= ra + rb
Factors affecting Bond length
1)Size of the atoms
The bond length increases with increase in size of the atom.
HI > HBr > HCl > HF
2)Multiplicity of Bond
The bond length decreases with the multiplicity of the bond.
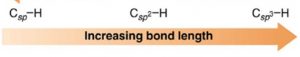
3)Type of hybridisation
An s orbital is smaller in size, greater the s character ,shorter is the hybrid orbitals and hence shorter is the bond length.
Bond enthalpy
When atoms come close together resulting in the formation of bond between them ,energy is released.
The amount of energy required to break one mole of bonds of a particular type so as to separate them into gaseous atoms is called bond dissociation enthalpy or Bond enthalpy.
Bond enthalpy is usually expressed in KJ mol-1
Greater is the bond dissociation enthalpy ,stronger is bond.
For diatomic molecules like H2 , Cl2, O2, N2, HCl, HBr, HI the bond enthalpies are equal to their dissociation enthalpy and hence have fixed. In case of polyatomic molecules, since a particular type of bond present in different molecules or even in the same molecule do not possess the same bond enthalpy, therefore , bond enthalpy are usually the average values.
In H20 , first O-H bond enthalpy=502 Kj/mol
Second bond enthalpy= 427 KJ/mol
Average bond enthalpy= (502 + 427 ) / 2 = 464.5 KJ/mol
Factors affecting bond enthalpy
1)Size of the atom
Greater the size of the atom ,greater is the bond length and less is the bond dissociation enthalpy i.e. less is the bond strength.
2)Multiplicity of bonds
Greater is the multiplicity of the bond, greater is the bond dissociation enthalpy.
3)Number of lone pair of electrons present
Greater the number of lone pair of electrons present on the bonded atom ,greater is the repulsion between the atoms and hence less is the bond dissociation enthalpy.
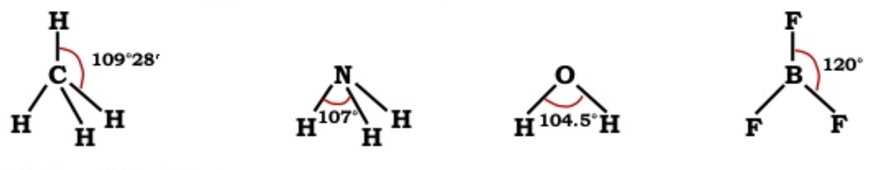
4)Bond angle
A bond is formed by the overlap of atomic orbitals. The direction of overlap gives the direction of the bond.
The angle between the lines representing the direction of the bond i.e. the orbitals containing the bonding electrons is called the bond angle.
5)Bond order
In lewis representation of a molecular or ion ,the number of bonds present between two atoms is called the bond order
For odd electron molecule, as the 3 electron bond is considered as equivalent to half covalent bond, the bond order can be fractional also.
Greater the bond order ,greater is the stability of the bond i.e. greater is the bond enthalpy.
Greater the bond order, shorter is the bond length.
Thanks for nice class notes. It’s really helpful for students
amazzzzzzingggggg