Question 1 Name few application of chemical effects of electric current?
Question 2 Name few compounds which are produced by using chemical effect of current?
Question 3 Name 2 metals which are purified by using the chemical effect of current?
Question 4 Describe how you will purify a strip of impure copper by using chemical effect of electric current?
Contents
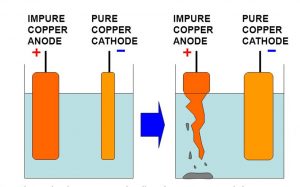
Purification of Metal
The chemical effects of electric current is used in the purification of impure metals.
In the purification of an impure metal by using the chemical effects of current:
(1) A thick rod of impure metal is made positive electrode or anode: It is connected to positive terminal of the battery.
(2) A thin strip of pure metal is made negative electrode or cathode: It is connected to the negative terminal of the battery.
(3) A water soluble salt of the metal to be purified is taken as electrolyte.
On passing electric current, the metal dissolves from the impure anode and goes into electrolyte solution. The metal present in dissolved form in electrolyte get deposited on the cathode in the pure form. The impurities are left behind in the electrolyte solution. The metals like Copper, zinc and Aluminium are purified by the process of electrolysis by using the chemical effects of electric current.
Activity : To purify impure copper metal
Take 250 ml of distilled water in a clean beaker. Dissolve two teaspoon full of copper sulphate in it. Add few drops of dilute sulphuric acid to copper sulphate solution. A thick rod of impure copper metal is made positive electrode by connecting it to the positive terminal of the battery. A thin plate of of pure copper metal is made negative electrode by connecting it to the negative terminal of the battery. Switch on the electric current by closing the switch. Allow the current to pass for about half an hour. It will be observed that the impure copper rod goes on becoming thinner and thinner. This is because the impure copper metal of anode goes on dissolving in copper sulphate solution where the pure metal from copper sulphate solution goes on depositing on copper plate cathode. Impurities present in impure rod of copper fall to the bottom of the beaker.
Production of Metals
The chemical effect of electric current is used in the production or extraction of certain metals from their naturally occurring compounds called ores.
The chemically reactive metals are produced by this method. The reactive metals such as sodium, aluminium and magnesium are produced by passing electric current through their compounds in molten state.
Sodium metal is produced by the electrolysis of molten sodium chloride whereas aluminium metal is produced by the electrolysis of molten Aluminium oxide.
Metal is produced at the negatively charged electrode this is because the positively charged ions of metal present in the molten compounds are attracted by the negatively charged electrode.
Production of Compounds
The chemical effect of electric current or electrolysis is used in the production of various chemical compounds. Sodium Hydroxide is produced by the electrolysis of an aqueous solution of sodium chloride.
Decomposition of Compounds
The chemical effect of electric current or electrolysis is used to decompose various chemical compounds into their elements.
For Example : Water can be decomposed by passing electric current into two elements hydrogen and oxygen.
Very nice answer thank you ma’am
thank you mam for your beautiful answer
Really useful for me…after a long time searching hopelessly in google I got it …Thank u so much….
Great work…I am very happy….Thank u to all the hands behind this…☺️☺️☺️
Thank you ma’am this was really helpful for me.