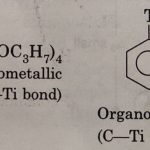Atomic Radii The atomic radii of the transition elements are intermediate between those of s- and p-block elements. (a) The atomic radii of elements of a particular series decrease with increase in atomic number but this decrease in atomic radii becomes small after midway. For example: For the elements of first transition series, the atomic radii decrease gradually … [Read more...] about Atomic Radii, Metallic Character, Enthalpy of Atomisation & Density of Transition Elements
Class 12
Characteristics and Electronic configuration of Transition Elements
Transition Elements The transition elements are those elements which have incompletely filled (partly filled) d-subshells in their ground state or in any one of their oxidation states. 1) The d-block elements are called transition elements. This block consists of elements lying between s- and p-blocks i.e. , between groups 2 and 13, starting from fourth period. 2) In … [Read more...] about Characteristics and Electronic configuration of Transition Elements
Organometallic Compounds
Organometallic Compounds Organometallic compounds are those compounds which contain one or more metal-carbon bonds. All the compounds containing carbon and a metal atom are not organometallic. We use this term for compounds which contain at least one M-C bond. For example An alkoxide such as (C3H7O4)Ti is not considered to be an organometallic compound because the … [Read more...] about Organometallic Compounds
Metal Carbonyls
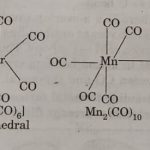
Metal Carbonyls There is another important class of coordination compound known as metal carbonyls in which carbon monoxide (CO) acts as ligand. These are also called homoleptic carbonyls (compounds containing carbonyl ligands only). These compounds contain both σ and π character. These are formed by many transition metals. Structure of Metal Carbonyls Homoleptic binary … [Read more...] about Metal Carbonyls
Method of Preparation and Importance of Coordination Compounds
Methods of preparation of Coordination Compounds Coordination compounds are generally prepared by common methods such as substitution reactions, redox reactions and by the direct combination of the reactants. (1) Substitution reactions The majority of complexes are prepared by substitution reactions. The general method of synthesis is to replace water molecules … [Read more...] about Method of Preparation and Importance of Coordination Compounds