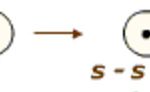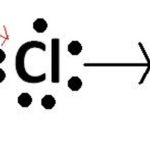Depending upon the type of overlapping, the covalent bonds are mainly of two types: Sigma bond (σ) When a bond is formed between two atoms by the overlap of their atomic orbitals along the internuclear axis, the bond formed is called sigma bond The overlapping along the internuclear axis can take place in any one of the ways: (1) s-s overlapping Overlapping … [Read more...] about Types of Covalent Bond
Class 11
Valence Bond Theory
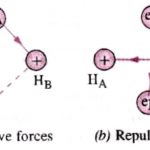
This theory was put forward by Heitler and London in 1927 and was further developed by Pauling and others. In terms of energy When the two atoms are far apart from each other there is no interaction between them. When they come closer to each other ,the new forces come into operation. These forces are of two types: 1)The forces of repulsion between the nuclei of … [Read more...] about Valence Bond Theory
Valence Shell Electron Pair Repulsion Theory
The first simple theory that was put forward to explain the shape of the molecule is known as Valence Shell Electron Pair Repulsion Theory. This theory was given by Sidgwick and Powell in 1940. 1) The central atom is linked to other atoms by covalent bonds which are formed by sharing of electrons. 2) The central atom is surrounded by a shared pair of electrons and … [Read more...] about Valence Shell Electron Pair Repulsion Theory
Covalent Bond
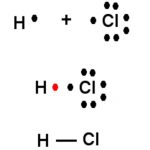
The bond formed between the two atoms by mutual sharing of electrons between them so as to complete their octets or duplets in case of elements having only one shell is called covalent bond and the number of electrons contributed by each atom is known as covalency. Examples Two chlorine atoms combine to produce chlorine molecule. Each of them contributes one electron to … [Read more...] about Covalent Bond
Ionic Bond
When a bond is formed by complete transference of electrons from one atom to another so as to complete their outermost orbit by acquiring 8 electrons or 2 electrons in case of hydrogen, lithium etc and hence acquire the stable nearest noble gas configuration, the bond form is called ionic bond or electrovalent bond. On losing an electron, an atom becomes positively charged … [Read more...] about Ionic Bond