In a liquid, the molecules are quite close together so that there are considerable forces of attraction between them and hence they are held together in a definite volume. The liquids possess fluidity like gases but incompressibility like solid. Properties of Liquid (1) Liquids have no definite shape: They take up the shape of the vessel in which they are put. This is … [Read more...] about Vapour Pressure
Class 11
Deduction of Gas Laws From kinetic Theory
Boyle's law At constant temperature, the average kinetic energy and hence the average speed of the molecules is constant. The number of molecules present in a given mass of a gas is also constant. Let the volume of a given mass of a gas be reduced to one half of its original volume. The same number of molecules with their same average speed will now have half the original … [Read more...] about Deduction of Gas Laws From kinetic Theory
Maxwell-Boltzmann Distribution
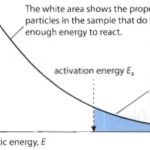
Maxwell-Boltzmann distribution of Molecular speed At a particular temperatures, different molecules of a gas possess different speeds. Due to continues collision among the molecules themselves and against the walls of the container ,their speed keep on changing. As a result of collision, some others are speeded up, some others are slowed down and hence the fashions of … [Read more...] about Maxwell-Boltzmann Distribution
Energy level diagram for Molecular orbitals
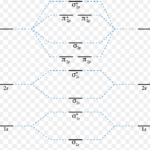
Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow: σ(1s) <σ∗(1s) < σ(2s) <σ∗(2s) < π(2px) = π(2py) < σ(2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) Relationship between electronic configuration and Molecular behaviour 1) Stability of molecules in terms of bonding and antibonding … [Read more...] about Energy level diagram for Molecular orbitals
Liquefaction of Gases And Critical Temperature
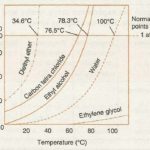
The liquefaction of a gas takes place when the intermolecular forces of attraction become so high that they bind the gas molecules together to form the liquid state. The intermolecular forces of attraction can be increased either by increasing the pressure so that the molecules come close together or by cooling the gas so that the kinetic energy of the molecules decreases … [Read more...] about Liquefaction of Gases And Critical Temperature