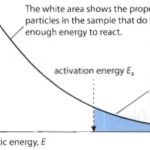
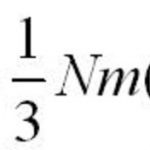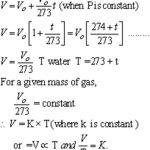Maxwell-Boltzmann distribution of Molecular speed At a particular temperatures, different molecules of a gas possess different speeds. Due to continues collision among the molecules themselves and against the walls of the container ,their speed keep on changing. As a result of collision, some others are speeded up, some others are slowed down and hence the fashions of … [Read more...] about Maxwell-Boltzmann Distribution
States of Matter
Liquefaction of Gases And Critical Temperature
The liquefaction of a gas takes place when the intermolecular forces of attraction become so high that they bind the gas molecules together to form the liquid state. The intermolecular forces of attraction can be increased either by increasing the pressure so that the molecules come close together or by cooling the gas so that the kinetic energy of the molecules decreases … [Read more...] about Liquefaction of Gases And Critical Temperature
Kinetic Molecular Theory Of Gas
Kinetic theory of gases Postulates or assumptions of kinetic theory of gases 1)Every gas is made up of a large number of extremely small particles called molecules. All the molecules of a particular gas are identical in mass and size and differ in these from gas to gas. 2)The molecules of a gas are separated from each other by large distances so that the actual volume … [Read more...] about Kinetic Molecular Theory Of Gas
Gas Laws
Boyle's law The relationship between the volume and pressure of a gas was studied experimentally by Robert Boyle in 1662. He used mercury and a simple U-tube. The pressure was increased by putting more mercury into the open limb. The volume of the air enclosed in the space above mercury in the shorter limb was noted each time. Boyle's law states that … [Read more...] about Gas Laws
Characteristics of Gases
Characteristics of Gases 1) Gases have neither definite shape nor definite volume. They take up the shape and volume of the container. 2) They have lower density than liquids and solids. 3) They are highly compressible 4) Gases intermix completely in all proportion without any mechanical aid. 5) They exert pressure equally in all direction. Measurement of … [Read more...] about Characteristics of Gases