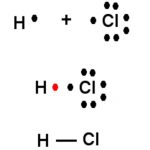
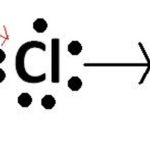The bond formed between the two atoms by mutual sharing of electrons between them so as to complete their octets or duplets in case of elements having only one shell is called covalent bond and the number of electrons contributed by each atom is known as covalency. Examples Two chlorine atoms combine to produce chlorine molecule. Each of them contributes one electron to … [Read more...] about Covalent Bond
Chemical Bonding and Molecular Structure
Ionic Bond
When a bond is formed by complete transference of electrons from one atom to another so as to complete their outermost orbit by acquiring 8 electrons or 2 electrons in case of hydrogen, lithium etc and hence acquire the stable nearest noble gas configuration, the bond form is called ionic bond or electrovalent bond. On losing an electron, an atom becomes positively charged … [Read more...] about Ionic Bond
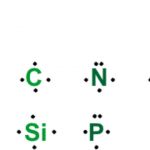
Lewis Symbols
A group of atoms existing together as one species and having characteristic properties is called a molecule. This force which holds the atoms together within a molecule is called a covalent bond. Noble gases neither combine chemically with any other element nor among themselves i.e. they are chemically inert. Noble gases are inactive or stable because they have 8 … [Read more...] about Lewis Symbols