Question 1 Define the term evaporation?
Question 2 Describe how will you separate common salt from sea water?
Question 3 Explain the process to recover only salt?
Question 4 State one large scale use of process of evaporation?
Question 5 Define the term evaporation and condensation?
Also Read NCERT Solutions for Chapter 5 Separation of Substances
Evaporation
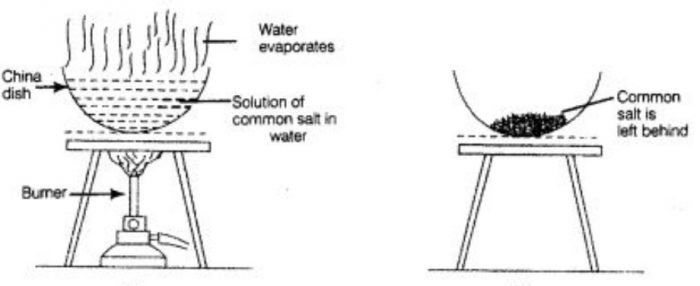
The changing of a liquid into vapours or gas is called evaporation. Evaporation is used to obtain a solid substance that has dissolved in water or any other liquid.
The dissolved substance is left as a solid residue when all the water has evaporated. The use of process of evaporation for separating a mixture is based on the fact that liquid vaporise easily whereas solids do not vaporise easily. Evaporation can be made quicker by heating the solution.
If we have a mixture of common salt and water, then we cannot separate salt from water by filtration. This is because common salt is completely dissolve in water and not insoluble in it. We can recover common salt from salt water mixture by the process of evaporation.
The solution of common salt and water is taken in a porcelain dish and heated gently by using a burner. The water present in salt solution will form water vapours and escape into atmosphere. When all the water present in the solution of common salt and water gets evaporated, then common salt is left behind in porcelain dish as a white solid.
The process of evaporation is used on a large scale to obtain common salt from seawater. Sea water is trapped in shallow lakes and allowed to stand there. The heat of Sun gradually evaporates water in the shallow lakes and and common salt is left behind as a solid. Sea water contains many salt dissolved in it. When sea water is evaporated we get a mixture of salt. Common salt is obtained from this mixture of salt by further purification.
When a sugar solution is evaporated, then water is eliminated and solid sugar is left behind. The substances like Potash alum and potassium nitrate are also separated from the water solution by the process of evaporation.
During the evaporation of a water solution we get the dissolved solid substance but water cannot be recovered in this method.
Distillation
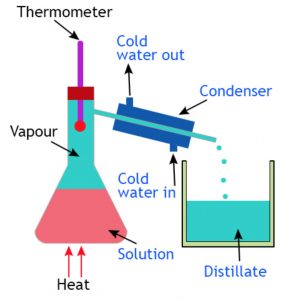
In order to recover both salt as well as water from salt water mixture the process of distillation is used.
Distillation is the process of heating water to form water vapours or steam, and then cooling the hot water vapours to get back liquid water water.
condensation
The changing of water into water vapours on heating is for evaporation or boiling. The changing of water vapours into liquid water on cooling is called condensation.
Distillation involves two processes evaporation followed by condensation.
The separation of a salt-water mixture into salt and water by distillation depends on the fact that water is a volatile liquid which forms vapour on heating but salt is a non volatile solids which does not forms vapour on heating.
Put the salt water mixture in a metal kettle and close its lid. Heat the kettle on a burner. After sometimes salt water mixture starts boiling and steam starts coming out from the spout of the kettle. Take a frying pan with a wooden handle and put some ice in it. Hold the frying pan in slanting position just above the spout of the kettle in such a way that steam coming out of the spout comes in contact with the bottom of frying pan.
When the hot steam comes in contact with the ice cold bottom of frying pan, it gets cooled and condenses to form drops of liquid water. This pure water is collected in a beaker kept below the frying pan. After all the water of salt water mixture taken in the kettle boils off, salt is left behind in the kettle. This salt can be taken out from the kettle. In this way, salt water mixture has been separated into salt and water by the process of distillation.
| Notes for Chapter 5 Separation of Substances |
Very nice explanation. If such types of websites are available on internet then there is no needs of tuitions for student. Please keep it up. It gives a lot of information
Nice explanation in simple language
Super
Well explained and detailed.
these websites are very useful.
So good
your information is so helpful. And can be understand easily because of clear words used in it