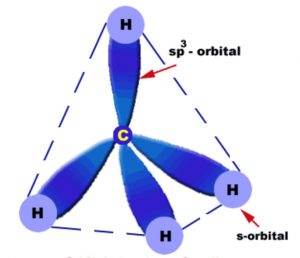
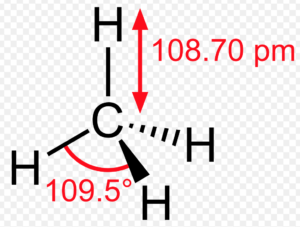
Methane molecule
A molecule of methane contains four C-C , σ bonds.
Orbital picture of methane
Bond length and bond angles
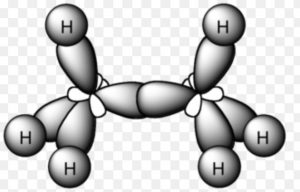
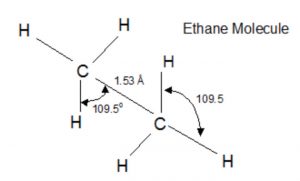
Ethane Molecule
It contains 6 C-H σ bonds and one C-C σ bond.
Orbital picture of ethane
Bond lengths and bond angle in ethane
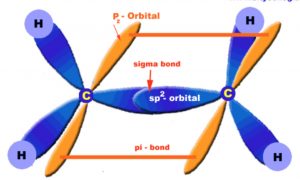
Ethene molecule
The carbon-carbon double bond of ethene consists of a strong carbon-carbon σ- bond and a weak carbon-carbon π bond.
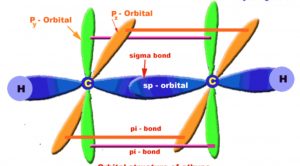
Ethyne molecule
The carbon-carbon triple bond of ethyne consists of a strong carbon-carbon σ- bond and two weak carbon-carbon π bond.
Characteristic Features of π-bonds
For π-bond formation, it is essential that the p-orbital on the adjacent carbon atoms must be parallel for a proper sideways overlap.
1) All the atoms directly attached to the carbon atoms of the double bond lie in the same plane.
2) The p-orbital of a π-bond are mutually parallel and are perpendicular to the plane of the molecule.
3) Rotation of one CH2 fragment w.r.t the other interferes with the maximum overlaps of the p-orbitals.Therefore , such a rotation about carbon-carbon double bond is restricted.
4) The electron charge cloud of the π-bond lies above and below the plane of the bonding atoms.This allows the π-electrons being easily available to the attacking reagents.
Thanks