Contents
Oxidation State
(1) Oxidation state of Carbon
The general valence shell electronic configuration of elements of group 14 is ns2 np2 where n is the number of outermost principal shell. These elements can attain inert gas configuration either by losing or gaining 4 electrons forming M4+ or M4- ions.
The C4+ ion does not exist firstly because it is a highly charged species and secondly its formation requires a very high ionisation enthalpy which is usually not available in chemical reactions.
Carbon can take up can take up 4 electrons to form carbide ion, C4- ion. Such a process is also energetically not favourable since the chemical species is highly charged and thus requires large amount of energy for adding 4 electrons.
(2) Oxidation state of other Elements
Silicon also shows an oxidation state of +4.The remaining elements of this group i.e. Ge, Sn and Pb show +2 and +4 oxidation state due to inert pair effect which arises due to ineffective shielding of the valance s electrons by the intervening d or f electrons.
As the number of d and f electron increases down the group from Ge to Pb ,the inert pair effect become more and more prominent. As a result, the stability of +4 oxidation state decreases while that of +2 oxidation state increases from Ge to Pb.
The stability of the +2 oxidation state increases markedly in the sequence : Ge < Sn <Pb i.e.oxidation state of lead is the most stable.
Covalent to ionic Character
Compounds of group 14 elements which show an oxidation state of +4 are expected to be covalent because of their extremely small size and high charge whereas compounds which show an oxidation state of +2 are expected to be ionic because of large size and small charge.
SnCl2 is an ionic solid while SnCl4 is a covalent liquid.
As we move down the group ,the tendency of the elements to form covalent compound decreases whereas the tendency to form ionic compounds increases.
Oxidising and reducing properties
Due to inert pair effect ,the elements Ge, Sn and Pb show two oxidation states of +2 and +4 therefore, these elements in +2 oxidation state can act as reducing agent while in +4 oxidation state they can act as oxidising agent.
Reducing agent
M2+ ———> M4+ + 2 e‾
Oxidising agent
M4+ + 2e‾ —–> M2+
Since +4 oxidation state of germanium is the most stable followed by Sn and Pb, therefore in group 14 ,Ge ( ) salts are the strongest reducing agent followed by Sn (
) salts.
Sn () salts such as SnCl2 is widely used as a reducing agent.
2 FeCl3 + SnCl2 ——-> 2 FeCl2 + SnCl4
2 HgCl2 + SnCl2 ——-> 2 Hg2Cl2 + SnCl4
Hg2Cl2 + SnCl2———–> 2 Hg + SnCl4
Since + 2 oxidation state of lead is the most stable followed by tin and germanium therefore, lead salts such as tetraacetate , Pb(OCOCH3)4 and PbO2 are widely used as oxidising agents.
PbO2 + 4 HCl ————> PbCl2 + Cl2 + 2 H2O
Tendency to form pπ-pπ bonds
Due to small size and higher electronegativity, carbon has a strong tendency to form multiple bonds either with itself or with the other atoms of similar size such as oxygen and nitrogen.
As we move down the group from carbon to lead, this ability to form pπ-pπ multiple bond decreases due to corresponding increase in size and decrease in electronegativity of the atom.
The reluctance of silicon to form pπ-pπ bonds to itself is shown by the following facts:
(1) Elemental silicon exist only in the diamond structure and not in the graphite structure and no form of elemental silicon is comparable to graphite.
(2) CO2 containing two C O double bond is a gas while SiO2 is a solid.It consists of an infinite three dimensional network of Si-O single bonds.
Tendency to form dπ-pπ bonds
Carbon does not have d-orbital and hence it does not form dπ-dπ bonds. Silicon and other heavier elements of this group because of the presence of vacant d-orbital in them tend to form dπ-pπ bonds. This tendency is particularly strong in case of silicon linked to oxygen and nitrogen.
For Ex: In trimethylamine both carbon and nitrogen are sp3 hybridised. Since nitrogen contains a lone pair of electrons, therefore, geometry around nitrogen is pyramid while that around carbon is tetrahedral.
In trisilylamine , N(SiH3)3 , N is sp2 hybridised. A sp2– hybridised nitrogen has a lone pair of electrons in a 2p-orbital which can overlap with an empty d-orbital of silicon to form dπ-pπ bonds. Additional bonding is responsible for change of hybridisation of nitrogen from sp3 to sp2 .As a result , N(SiH3)3 has trigonal planar geometry.
Due to dπ-pπ bonding the lone pair of electrons is transferred from nitrogen to silicon. As a result, N(SiH3)3 is a weaker base than (CH3)3 N.
Maximum covalency and tendency to form complexes
Carbon because of the absence of d-orbitals, cannot expand its valence shell and hence its maximum covalency or coordination number is 4.
Si, Ge , Sn and Pb due to the availability of vacant d-orbital show a coordination of greater than 4 forming pentacoordinated and hexacoordinated complexes.
[SiF5]‾ , [SiF6]‾ , [GeCl6]2- , [Sn(OH)6]2- , [Pb(OH)6]2- , [PbCl6]2-
In these complexes hybridisation of central atom is sp3d2.
Due to the presence of d-orbital , the tetrahalides of Ge, Si , Sn , Pb undergo hydrolysis.
Reactivity towards oxygen
All the elements of group 14 when heated in oxygen from oxides. These are mainly of two types i.e. monoxide (MO) and dioxide(MO2).
SiO is formed by reduction of SiO2 with Si i.e.
SiO2 + Si ——–> 2 SiO
lead also forms another oxide called trilead tetroxide or red lead. It is obtained by heating PbO with excess of air or oxygen at 673 K.
6PbO + O2 —-> 2 Pb3O4
Properties
(1) Acid base character: The oxides in higher oxidation state of the elements are generally more acidic than those in the lower oxidation state. As we move down the group ,the acidic character decreases. Thus among dioxides, CO2 and SiO2 are acidic, GeO2 is less acidic whereas SnO2 and PbO2 are amphoteric.
Being acidic CO2 , SiO2 and GeO2 react only with bases.
CO2 + 2NaOH —-> Na2CO3 + H2O
CO2 + Ca(OH)2 ——> CaCO3 + H2O
SiO2 + 2NaOH —-> Na2SiO3 + H2O
GeO2 + 2NaOH —-> Na2GeO3 + H2O
SnO2 and PbO2 react with both acids and bases.
SnO2 + 2NaOH —-> Na2SnO3 + H2O
SnO2 + 4HCl —-> SnCl4 + 2H2O
PbO2 + 2NaOH —-> Na2PbO3 + H2O
PbO2 + 4HCl —-> PbCl4 + 2H2O
CO is neutral , GeO is distinctly acidic whereas SnO and PbO are amphoteric.
(2) Reducing- Oxidising power : Since +4 state of carbon is most stable, therefore, among the monoxides of group 14 , CO is the strongest reducing agent. Therefore it is used in the extraction of many metals from their oxides.
Fe2O3 + 3 CO ——–> 2 Fe + 3 CO2
ZnO+ CO —–> Zn + CO2
Due to inert pair effect, +2 oxidation state of lead is the most stable, therefore ,among dioxides of group 14, PbO2 is a powerful oxidising agent.
It oxidises HCl to Cl2 and reacts with conc HNO3 or H2SO4 to evolve O2.
2 PbO2 + 4 HNO3 ——–> 2 Pb(NO3)2 + 2 H2O + O2
2 PbO2 + 2 H2SO4 —–> 2 2 PbSO4 + 2 H2O + O2
PbO2 + 4 HCl ——-> PbCl2 + Cl2 + H2O
Reactivity towards water
Carbon, Silicon and Germanium do not decompose water at all. Tin decomposes steam to form tin dioxide and dihydrogen gas.
Sn (s) + 2 H2O (g) ———> SnO2 (s) + 2 H2 (g)
Lead is not affected by water due to the formation of protective film of lead oxide on its surface.
Reactivity towards halogens
The elements of group 14 form halides of the formula MX4 and MX2.
Tetrahalides
(1) All the elements of group 14 form tetrahalides of the formula MX4.
(2) Most of the tetrahalides are covalent in nature. The central atom in these halides undergo sp3 hybridization and the molecule is tetrahedral in shape.
SnF4 and PbF4 are ionic in nature.
(3) The thermal stability of these halides decreases with the increasing atomic number or the size of the halogen atom.PbCl4 is stable, PbBr4 is unstable while PbI4 is unknown
The non-existence of PbI4 may also be explained as:
The Pb-I bond initially formed during the reaction does not release enough energy to unpair 6s2 electrons and excite one of them 6 p-orbital to have four unpaired electrons around lead atom.
(4) The tetrachloride of carbon (CCl4) is not hydrolysed by water. The tetrachloride of all the remaining elements are easily hydrolysed.
SiCl4 + 4 H2O ——–> Si(OH)4 + 4 HCl
SnCl4 + 2 H2O —-> SnO2 + 4 HCl
CCl4 is not hydrolysed by water because carbon does not have d-orbital and hence cannot expand its coordination number beyond 4. Silicon can expand its octet due to the availability of energetically suitable vacant d-orbital in its atoms.
CCl4 + H2O ——–> No reaction
SiCl4 + 4 H2O ——> Si(OH)4 + 4 HCl
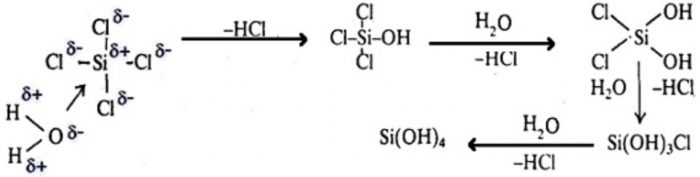
The mechanism of hydrolysis of SiCl4 involves the the following steps:
Step 1 : It involves nucleophilic attack by lone pair of electrons present on the oxygen atom of the water molecules on the metal atom forming a co-ordinate bond between the metal and oxygen atom of the water.
Step 2 : It involves the loss of HCl. During this step, one chlorine atom of silicon in SiCl4 is replaced by an OH group. This process continue till all the four chlorine atoms are replaced by OH group yielding Si(OH)4 i.e. silicic acid.
(5) The tetrahalides of carbon do not form complexes because carbon does not have vacant d-orbitals in its valence shell and hence cannot increase its coordination number beyond 4. Tetrahalides of other elements form complexes due to the availability of vacant d-orbital in their respective vacant shell. Therefore, these can increase their coordination number to 6. Tetrahalides of Ge, Sn and Pb behave as Lewis acids but tetrahalides of carbon does not.
SiF4 + 2 HF ——-> H2SiF6
SnCl4 + 2 Cl‾ ——-> SnCl62‾
Dihalides
All the elements, except carbon and silicon form dihalides , MX2 .
The stability of these dihalides increases steadily due to inert pair effect as we move down the group from Ge to Pb , i.e. GeX2 << SnX2 << PbX2 .
GeX4 is more stable than GeX2 whereas PbX2 is more stable than PbX4.
Excellent very useful
Thanx i learnt more
very useful notes
Very useful, I really like it