Beryllium shows resemblance with its diagonally opposite element aluminium.
Points of similarity are:
(1) Both metals have a tendency to form covalent compounds e.g. the chlorides of both being covalent are soluble in organic solvents.
(2) Both BeCl2 and AlCl3 act as a Lewis acids and are used as Friedel – Crafts catalyst.
(3) These two elements have same electronegativity and the polarising power i.e. charge/ radius ratio of their ions are very similar.
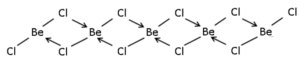
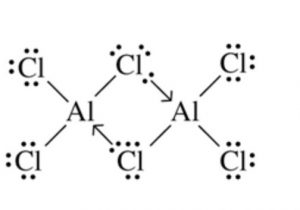
(4) BothBeCl2 and AlCl3 have chlorine bridged structures in the vapour phase.
(5) Both Be and Al are resistant to the action of acids due to the formation of protective film of the oxides on their surface.
(6) Both the metals dissolve in strong alkalies to form soluble complexes: beryllates and aluminates.
(7) The oxides of both beryllium(BeO) and Aluminium(Al2O3) are hard, high melting insoluble solids.
(8) The oxides and hydroxides of both Be and Al are amphoteric and dissolve in sodium hydroxide solution as well as in hydrochloric acid.
BeO + 2 HCl → BeCl2 + H2O
BeO + 2 NaOH → Na2BeO2 + H2O
Al2O3 + 6 HCl → 2 AlCl3 + 3 H2O
Al2O3 + 2 NaOH → 2 NaAlO2 + H2O
(9) Carbides of both the metals react with water liberating methane gas.
(10) Salts of both these elements form hydrated ions e.g.[ Be(OH)4]2+ and [Al(H2O)6]3+ in aqueous solution.
(11) Because of similar polarising power both beryllium and Aluminium form complexes. For eg: beryllium forms tetrahedral complexes such as [BeF4]2– and Aluminium forms octahedral complexes like [AlF6]3-
This is good work, thanx to rthe innovation.
Is it possible to download this content
Welcome.yes u can download the content.
This a nice work
Thanks neeta.
Good and correct information
Good useful information
Thank’s Mrs Shilpi it was of big help
Actually information is very useful
thank you.it was very helpful
This is very helpful information indeed thank you,u just narrowed down my research
This was really helpful information thank u
It had helped me to do my course work here at Kyambogo University.
Thank u so much ma’am giving this information …….
My problem has been solved
Thanks
At makerere university, Uganda we appreciate your service madam.
Thanks mam
Thank you so much ma’am your stuff is so powerful. ..keep the good work ..much love
We really appreciate ma’am
Understanding language . good explain
Thanks for giving correct as well as best information
These work is very much good and even the information in these website is also contains correct and point to point information . thank u very much mam for information. by writing these answer in my examination paper. I have achieved full marks.
thankyou so much ,i learnt a lot
the information given here is precise and straight …am student of chemistry in kymbogo university uganda
This answer is very useful thanks for the answer
From Department of education chemistry university of calabar,Nigeria.we thank u and say well done ma, may our good GOD reward the work of ur hands
Thank-you so much! This was short but very informative and correct content too
Thanks again
Thank you so much! This was short but very informative and correct content too
thank you soo much it was really helpfull
Thanks I’m scholar at St Joseph University taking Bsc physics and chemistry I real appreciate this work as helping most of scholars
Thanks much for my problems have been answered thanks again
Nice and simple concepts to understand, thanks for the good work!
I find this very helpful. Thank you