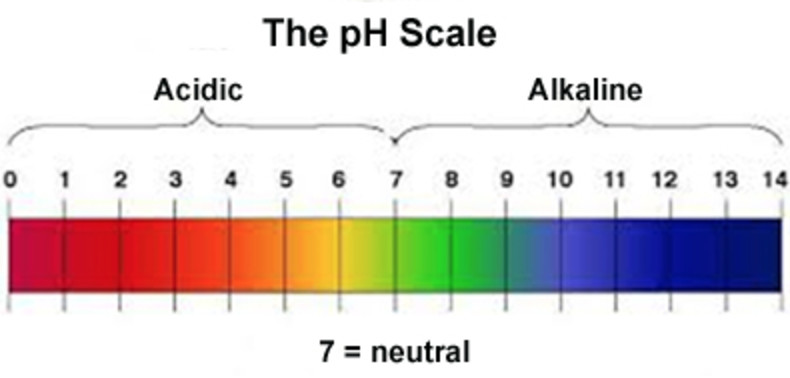
A solution may be neutral, acidic or alkaline.
Kw = 1 × 10-14 at 298 K
To express the acidity or alkalinity of a solution, it is sufficient to express only the H3O+ ion concentration.
Sorensen , in 1909 , suggested a convenient method of expressing the H3O+ ion concentration in terms of pH.
The symbol has been taken from the Danish word potenz de hydrogen ion which mean power of hydrogen ion.
pH of a solution is defined as negative logarithm of hydrogen ion concentration.
pH scale
The pH scale range is taken as 0 to 14 for practical purpose.
Relationship between pH and pOH
Relationship between Ka and Kb or pKa and pKb
log kw = log ka + log kb
– log kw = –log ka – log kb
–log ka – log kb = – log 10-14
pKa + pKb = pKw = 14
Limitation of pH scale
The pH value of the solutions do not give the exact idea of their relative strength
Measurement of pH
Accurate measurement of pH of a solution is done with the help of an instrument , called pH- meter.
Approximate pH can be determined with the help of pH papers which show different colours when dipped in solution of different pH.

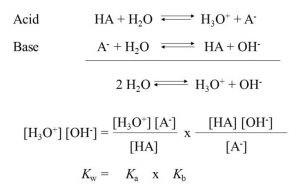
Thank you very much for this post (ph class 11), thanks a lot
Really proved to be very useful..
To be honest best answer