An electrolyte is defined as a compound whose aqueous solution or melt conduct electricity.
A compound whose aqueous solution or melt does not conduct electricity is called a non-electrolyte.
Aqueous solution of inorganic acids, bases and salts conduct electricity and hence they are electrolytes.
Aqueous solution of sugar, urea do not conduct electricity and hence they are non electrolytes.
The conductance by an electrolyte is due to the presence of ions produced by the dissociation of the substance. Different electrolytes dissociates to different extents.
The fraction of the total number of molecules which dissociate into ions is called the degree of dissociation or degree of ionization.
It is represented by α.
α= No. of moles dissociated / Total no. of moles taken
Depending upon the degree of dissociation, Faraday, further classified the electrolytes into two categories:
1) Strong Electrolytes
A strong electrolyte is defined as a substance which dissociates almost completely into ions in aqueous solution and hence is a very good conductor of electricity.
For Ex: NaOH , KOH, HCl, H2SO4 , NaCl
HCl + H2O ———> H3O+ + Cl−
NaOH + aq ———> Na+ (aq ) + OH– ( aq )ionisation constant
2) Weak Electrolyte
A weak electrolyte is defined as a substance which dissociates to small extent in aqueous solution and hence conduct electricity also to a small extent.
For Ex: NH4OH , CH3COOH
CH3COOH + H2O CH3COO‾ + H3O+
NH4OH + aq NH4+ ( aq ) + OH‾ (aq )
The ionisation of a weak electrolyte , AB , is represented as :
AB ( s ) + aq A+ (aq ) + B‾ ( aq )
Such and equilibrium is called Ionic Equilibrium between the ions and the undissociated electrolyte.
Applying the law of chemical equilibrium to the above equation:
where K is the ionisation constant.
When an ionic compound is dissolved in water ,the ions which are already present in the solid compound separates out. The process is called dissociation.
When a neutral molecule which does not contain ions but when dissolved in water splits to produce ions in the solution, the process is called ionization.
An ionic compound is a cluster of positively and negatively charged ions held together by electrostatic force of attraction. When such an ionic compound is put into water ,the high dielectric constant of water reduces the electrostatic forces of attraction. Thus, ions become free to move in the solution.
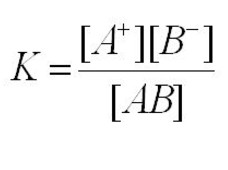
Leave a Reply