The branch of science which deals with the study of different forms of energy and the quantitative relationship between them is known as Thermodynamics.
Importance of thermodynamics
1)It helps us to predict whether any given chemical reaction can occur under the given set of conditions.
2)It helps in predicting the extent of reaction before the equilibrium has attained.
3)It helps to deduce some important laws like laws of chemical equilibrium, distribution law .
Limitations of thermodynamics
1)It helps to predict the feasibility of a process but does not tell anything about the rate at which the process take place.
2)It deals only with the initial and final states of a system but does not tell anything about the mechanism of the process.
3)It deals with the properties like temperature ,pressure of the matter in bulk but does not tell anything about the individual atoms and molecules.
Some basic terms and concepts
1)System and Surrounding : The path of Universe chosen for thermodynamic consideration is called a system.
The remaining portion of the universe ,excluding the system, is called surrounding.
Universe = system + surrounding
A system usually consists of a definite amount of one or more substance and is separated from the surrounding by a real or imaginary boundary through which matter and energy can flow from the system to the surrounding or vice versa.
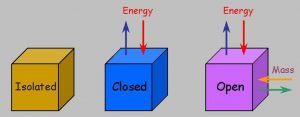
2)Open ,closed and isolated system
Open system : A system is said to be an open system if it can exchange both matter and energy with the surrounding.
For ex: If some water is kept in an open vessel or if some reaction is allowed to take place in an open vessel ,exchange of both matter and energy takes place between the system and surrounding.
Closed system : If a system can exchange only energy with the surrounding but not matter it is called a closed system.
For ex: If some water is placed in a closed metallic vessel or if some reactions is allowed to take place in a cylinder enclosed by a piston ,then as the vessel is closed, no exchange of matter between the system and surrounding can take place.As the vessel has conducting walls, exchange of energy can take place between the system and the surrounding.
If the reaction is exothermic ,heat is given by the system to the surrounding.
If the reaction is endothermic ,heat is given by surrounding to the system.
If the reaction is accompanied by decrease in volume ,mechanical work is done by the surrounding on the system.
If the reaction is accompanied by increase in volume ,the mechanical work is done by the system on the surrounding.
Isolated system :If a system can neither exchange matter no energy with the surrounding ,it is called an isolated system.
For Ex: If water is placed in a vessel which is closed as well as insulated, no exchange of matter or energy can take place between the system and the surrounding.
State of a system
The state of a system means the condition of the system which is described in terms of certain observable properties such as temperature, pressure ,volume of the system.
If any of these properties of the system changes ,the system is said to be in different state i.e. the state of system changes.
These properties of a system are called state variables.
The first and the last State of a system are called the initial state and the final state.
State function
A physical quantity is said to be state function if its value depends only upon the state of the system and does not depend upon the path by which this state has been attained.
or
A physical quantity is said to be a state function if the change in its value during the process depends upon initial state and final state of the system and does not depend upon the path or route by which this change has been brought about.
For ex: Physical quantities which are state function include pressure ,volume, temperature ,internal energy enthalpy, entropy ,free energy etc.
Macroscopic system and macroscopic properties
If a system contains a large number of chemical species i.e. atoms, ions or molecules, it is called macroscopic system.
Thermodynamic does not deal with the properties of the individual atoms and molecules but deals with the matter in bulk. Properties of the macroscopic systems like temperature ,pressure ,volume, density ,melting point ,boiling point are called macroscopic properties or thermodynamic properties.
Macroscopic properties are of two types:
1)Extensive properties: These are those properties which depend upon the quantity of the matter contained in the system.
For ex: Internal energy, mass, volume, heat capacity ,enthalpy ,Gibbs free energy.
2) Intensive properties : These are those properties which depends only upon the nature of the substance and are independent of the amount of substance present in the system.
For ex: Temperature, pressure ,viscosity ,density ,surface tension, freezing point, boiling point, mole fraction etc.
An extensive property may become intensive property by specifying unit amount of the substance concerned. Mass and volume are extensive property but density and specific volume are intensive properties of the substance or the system.
Heat capacity is an extensive property but specific heat capacity is intensive.
Thermodynamic Equilibrium
A system is said to be in thermodynamic equilibrium if its macroscopic properties like temperature ,pressure do not change with time.
Thermodynamic process
A thermodynamic process is said to occur when the system changes from one state to another.
1)Isothermal process:
When a process is carried out in such a manner that the temperature remains constant throughout the process, it is called isothermal process. When such a process occur, heat can flow from the system to the surrounding and vice a Versa in order to keep the temperature of the system constant.
2)Adiabatic process:
When a process is carried out in such a manner that no heat can flow from the system to the surrounding or vice a versa i.e. the system is completely insulated from the surrounding, it is called adiabatic process.
3)Isochoric process
It is a process during which the volume of the system is kept constant.
4) Isobaric process
It is a process during which the pressure of the system is kept constant.
Reversible process
A process which is carried out infinitesimally slowly so that all changes occurring in the direct process can be exactly reversed and the system remains almost in a state of equilibrium with the surrounding at every stage of the process.
A process which is not carried out infinitesimally slowly so that the successive steps of the direct process cannot be retraced and any change in the external condition disturbs the equilibrium.
Leave a Reply