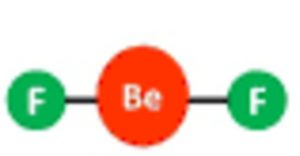
Shape of beryllium fluoride( BeF2) molecule
Atomic number of Be = 4
Electronic configuration in ground state is 1s2 2s2
Electronic configuration in excited state is 1s2 2s1 2px1
one 2s orbital and one 2p orbital undergo sp hybridisation to form two half filled sp hybrid orbitals which are oriented at an angle of 180°.
They overlap with the half filled orbitals of the two fluorine atoms to give a linear shape.
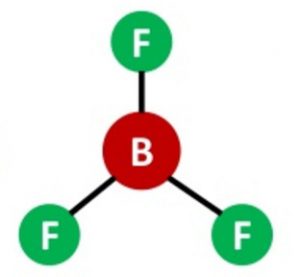
Shape of Boron trifluoride(BF3) molecule
Atomic number of Boron=5
Electronic configuration in ground state 1s2 2s2 2px 1
Electronic configuration in excited state = 1s2 2s1 2px1
One 2s and two 2p orbitals undergo sp2 hybridisation to form 3 half filled hybrid orbitals which are planar and oriented at an angle of 120° to each other. These overlap with half filled orbitals of 3 fluorine atoms to form BF3 which has triangular planar shapes.
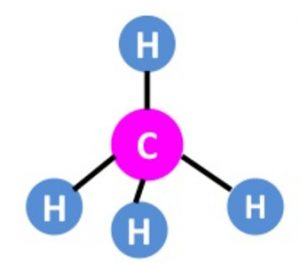
Shape of Methane ( CH4 )molecule
Atomic number of carbon = 6
Electronic configuration in the ground state is 1s2 2s2 2px 1 2py1
Electronic configuration in the excited state is 1s2 2s1 2px1 2py1 2pz1
One 2s and three 2p orbitals undergo sp3 hybridisation to form four sp3 hybrid orbitals which are arranged tetrahedrally at an angle of 109°28´to each other.
The 4 sp3 hybrid orbitals overlap with the half filled 1s orbital of 4 hydrogen atoms ,forming CH4.
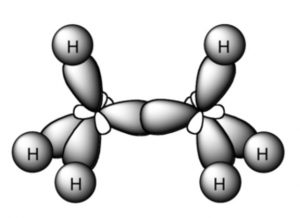
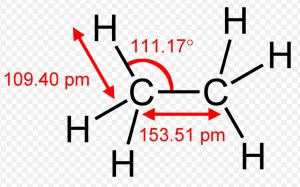
Shape of Ethane molecule(C2H6)
In the formation of ethane molecule, each carbon atom undergoes sp3 hybridisation ,thus forming four sp3 hybrid orbitals directed towards the corners of a tetrahedron and inclined to each other at an angle of109°28.
One sp3 hybrid orbital of the first carbon atom undergoes overlapping with one sp3 hybrid orbitals of second carbon atom along the internuclear axis ,thus forming a sigma bond between them.
The remaining 3 sp3 hybrid orbitals of each carbon atom undergo overlapping with the half filled 1s orbital of hydrogen atom, each along internuclear axis and hence forming sigma bond.
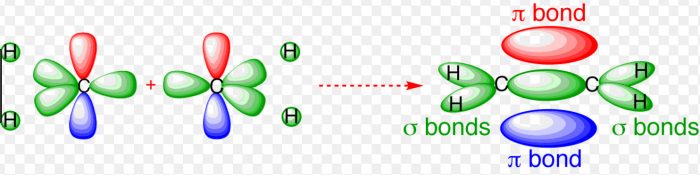
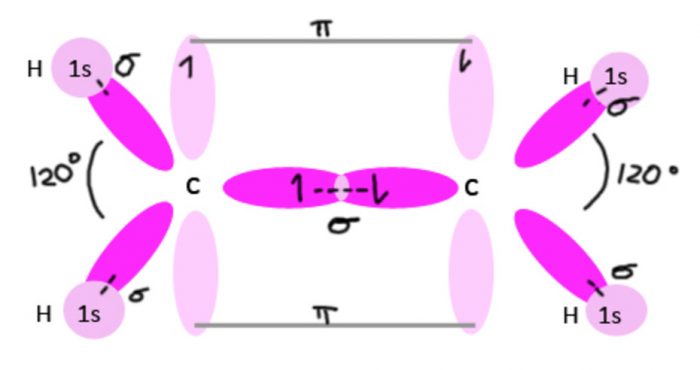
Shape of ethylene molecule (C2H4)
The electronic configuration of carbon atom in the excited state is 1s2 2s1 2px1 2py1 2pz1
Each carbon atom undergoes sp2 hybridisation, thus leaving one 2pz orbital is the unhybridized state.
The 3 sp2 hybrid orbitals of each carbon atom are planar and are inclined to each other at an angle of 120°.
One sp2 hybrid orbital of the the first carbon atom overlaps with one sp2 hybrid orbital of the second carbon atom along the internuclear axis thereby forming one sigma bond between them.
The other two sp2 hybrid orbitals of each carbon atom overlap with the half filled 1s orbital of hydrogen atom along their respective internuclear axis forming sigma bond.
The unhybridized 2pz orbital of the first carbon atom undergoes sideways overlapping with the unhybridized 2pz orbitals of the second carbon atom, thereby forming a π bond between the two carbon atoms. The two carbon atoms are linked to each other by 1 sigma bond and 1 π bond and each carbon atom is further linked to two hydrogen atoms by sigma bond. The molecule is planar.
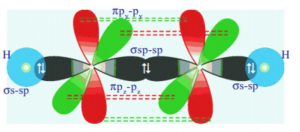
Shape of acetylene molecule
In the formation of acetylene molecule, each carbon atom undergoes sp hybridization leaving two 2p orbitals in the original unhybridised state. The two sp hybrid orbitals of each carbon atom are linear i.e. they are 180° degree apart.
One sp hybrid of the first carbon atom overlaps with one sp hybrid orbital of the second carbon atom along the internuclear axis thus forming sigma bond between them. The second sp hybrid orbital of each carbon atom overlaps with half filled 1s orbital of hydrogen atom again along the internuclear axis and thus forming Sigma bonds.
The unhybridised 2py orbital of the first carbon atom undergoes sideways overlapping with the 2py orbital of the second carbon atom, thereby forming a π bond between the two carbon atoms.
All the carbon and hydrogen atoms are linear and there is electron cloud above and below, in the front and at the back of the C-C axis.
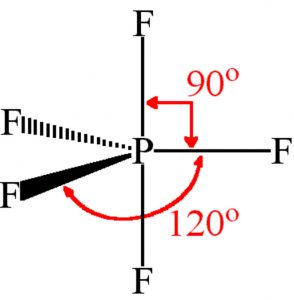
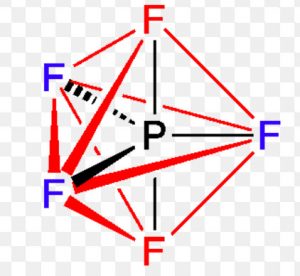
Shape of Phosphorus pentafluoride (PF5)
In Phosphorus pentafluoride molecule, the central atom, Phosphorus, has the ground state electronic configuration 1s2 2s2 2p6 3s2 3px1 3py1 3pz1 .
One of the 3s electrons get promoted to 3d orbital giving the electronic configuration1s2 2s2 2p6 3s1 3px1 3py1 3pz1 3d1.
It involve Sp3d hydridisation. Three of the fluorine atoms lie in the same plane as phosphorus atoms and are called equatorial fluorine atoms and the bonds formed are called equatorial bonds .The other two fluorine atoms lie at right angle to the plane of equatorial bonds and are known as axial fluorine atoms and the bond formed by them are called axial bonds.
The equatorial bonds are at an angle of 120° with each other whereas axial bonds make an angle of 90° with the equatorial bonds.
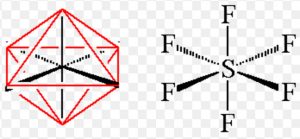
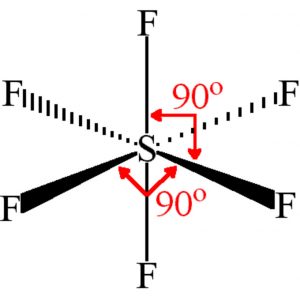
Shape of sulphur hexafluoride(SF6)
In SF6 molecule, central atom ,sulphur has the ground state electronic configuration 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 and fluorine has 1s2 2s2 2px2 2py2 2pz1. One of the 3s electrons as well as 3p electron get promoted to 3d orbitals giving the electronic configuration 1s2 2s2 2p6 3s1 3px1 3py1 3pz1 3d2.
Thus it involve sp3d2 hybridisation. The half filled hybrid orbitals overlap with the half filled orbitals of fluorine to form SF6. The central atom is surrounded by 6 bond pairs of electrons in the valence shell.
For SF6 to have minimum energy, the bond pair of electrons should be as far apart as possible from each other.The molecule acquires a regular octahedron.
In SF6, four S-F bonds are in the same plane at right angle to one another and are directed towards the four corners of a square. The other two fluorine atoms lie at right angle above and below the plane of F atoms.
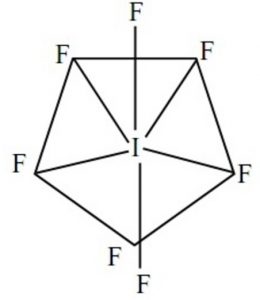
Shape of iodine hexafluoride molecule (IF7)
The electronic configuration of iodine is 5s2 5px2 5py2 5pz1 .
In the excited state, one electron each from 5s, 5px and 5py jumps to empty 5d orbital so that excited state configuration is 5s1 5px1 5py1 5pz1 5dxy1 5dyz1 5dxz1
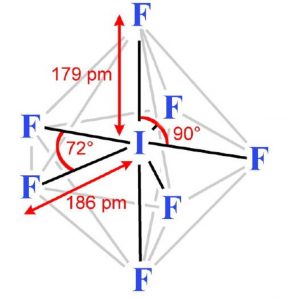
These 7 orbitals hybridise together to form seven sp3d2 half filled hybrid orbitals. Five of these are directed towards the corners of a regular pentagon thereby making an angle of 72° with each other. The remaining two are at right angles to the plane of the pentagon, one above the plane and the other below it.
Each of these half filled hybrid orbitals overlap with the half filled 2p orbitals of fluorine atom to form IF7 which has pentagonal bipyramidal shape.
As 5 equatorial I-F bonds form an angle of 72° with each other whereas the remaining two axial I-F bonds make an angle of 90° with the equatorial bonds, the 7 bonds are not equivalent.
Shape of Ammonia molecule
Atomic number of nitrogen=7
Its electronic configuration is 1s2 2s2 2px1 2py1 2pz1
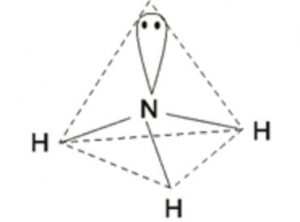
one 2s and three 2p orbitals undergo sp3 hybridisation forming 4 sp3 hybrid orbitals out of which 3 contains one electron each and take part in bond formation and fourth sp3 hybrid orbitals contains a lone pair of electrons and hence cannot participate in the bond formation.
The 4 sp3 hybrid orbitals will be directed towards the corners of a tetrahedron and hence the bond angle between any two sp3 hybrid orbitals would be 109°28´ .
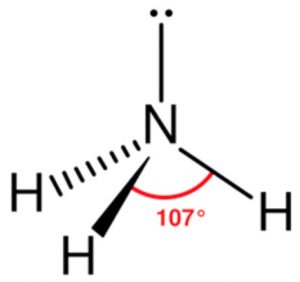
The bond angle in ammonia is found to be 107° .The decrease in angle is due to lone pair bond pair repulsion. Ammonia has trigonal pyramidal shape in which the three hydrogen atoms form the triangular base of the pyramid with nitrogen atom at the apex.
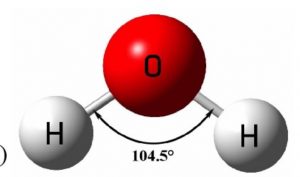
Shape of water molecule
Atomic number of oxygen is 8
Its electronic configuration is 1s2 2s2 2px2 2py1 2pz1
one 2s and three 2p orbitals undergo sp3 hybridisation forming four sp3 hybrid orbitals out of which 2 contains one electron each and the other two contains a pair of electrons.The four sp3 hybrid orbital thus found are directed towards the corners of a tetrahedron and hence the bond angle between any two sp3 hybrid orbitals would be 109°28´.
When the two sp3 hybrid orbitals containing one electron each, overlap with the half-filled 1s orbital of hydrogen atom to form water, the expected H-O-H bond angle is 109°28´. But experimentally the bond angle is found to be 104°.The lesser bond angle in water is due to two lone pair of electrons present which repel the bond pair.
Water is V shaped molecule or bent molecule.
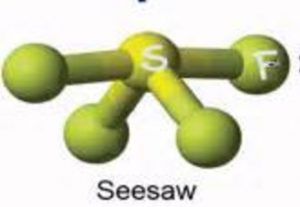
Shape of SF4 molecule
Atomic number of the central atom is 16
Its electronic configuration is 1s2 2s2 2p6 3s2 3px2 3py1 3pz1
In the excited state, it is 1s2 2s2 2p6 3s2 3px1 3py1 3pz1 3d1
It undergoes sp3d hybridisation to form 5 hybrid orbitals to give a trigonal bipyramidal arrangement in which one hybrid orbital occupies a lone pair and remaining four contains one electron each which are shared with those of fluorine atoms.
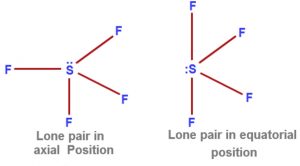
Depending upon the position occupied by the Lone pair ,two structure of SF4 are possible.
1)There are three lone pair – Bond pair repulsions at 90°
2)There are only two lone pair – Bond pair repulsion at 120°.
2nd structure is more stable.
It is distorted tetrahedron or folded square or a SeeSaw.
This is because the lone pair present at the equatorial position repels the bond pair present in the axial position.
Effect of electronegativity of the central atom on the bond angle of similar molecules
O, S, Se and Te undergo sp3 hybridisation forming 4 hybrid orbitals ,2 of which are occupied by lone pair of electrons.The expected bond angle is 109°28´.But the lone pairs repel the bond pairs thereby decreasing the angle.
Due to smaller size and high electronegativity of oxygen atom the bond pairs are closer to oxygen atoms and hence the repulsions between them are large. As we move from oxygen to Te , the size of the atom increases and electronegativity decreases. As a result the bond pairs move away from the central atom. The repulsion between the bond pairs decreases and so the bond angle also decreases.
In case of hydrides of group 15 ,the bond angle decreases as:
NH3 > PH 3 > AsH3 >BiH3
107.5° 93.4° 91.5° 91.2°
As the electronegativity of the central atom decreases, and the size increases, the bond angles decreases.
In the trihalides of phosphorus, the bond angle decreases as:
PF3 < PCl3 < PBr3 < PI3
97° 100° 101.5° 102°
Due to high electronegativity of fluorine ,bond pair of electron is more attracted towards F i.e. it lies away from the central atom. As electronegativity decreases from F to I, the bond pairs are closer to the central atom and the repulsion between the bond pairs increases and so the bond angle increases also increases.
As the electronegativity of the surrounding atoms decreases, the bond angles increases.
Leave a Reply