Hydrogen Spectrum
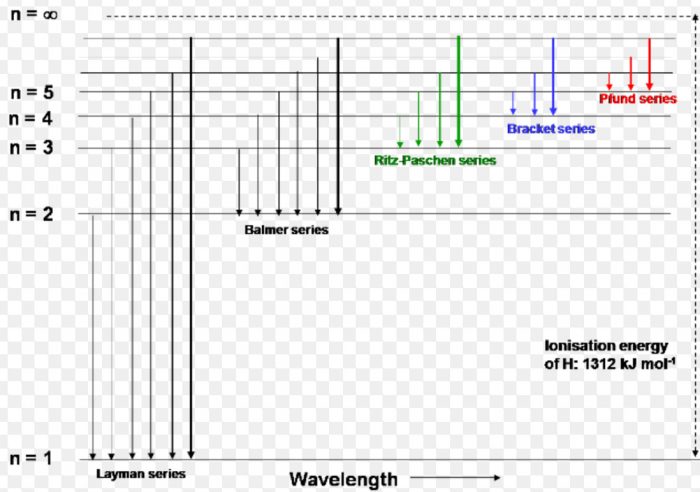
Atomic spectrum of hydrogen consists of a number of lines which have been grouped into 5 series :Lyman, Balmer, Paschen, Brackett and Pfund.
Any given sample of hydrogen gas gas contains a large number of molecules. When such a sample is heated to a high temperature or an electric discharge is passed, the hydrogen molecules splits into hydrogen atoms. The electrons in different hydrogen atoms absorb different amount of energies and are excited to different energy levels. Since the lifetime of electron in these excited states is very small, they return to some lower energy level or even to the ground state in one or more jumps.
Different excited electrons adopt different routes to return to various lower energy levels or the ground state. As a result, they emit different amount of energies and thus produce a large number of lines in the atomic spectrum of hydrogen.
(1) When the electron jumps from energy level higher than n=1 ie. n=2,3,4,5,6 ….to n=1 energy level, the group of lines produced is called lyman series. These lines lie in the ultraviolet region.
(2) The group of lines produced when the electron jumps from 3rd, 4th, 5th or any higher energy level to 2nd energy level, is called Balmer series. These lines lie in the visible region.
(3) Paschen series is obtained by the electronic jump from 4th, 5th or any higher energy level to 3rd energy level.
(4) Brackett series results from electronic transition from 5th, 6th or any higher energy level to 4th energy level.
(5) Pfund series originates by electronic jump from 6th, 7th or any higher energy level to 5th energy level. The spectral line of the last 3 series lie in the infrared region.
Can u please give me the all lines in spectrum in one diagram of all grades
Yes
That’s perfect
It is extremely good
This is what our teacher taught us.
That was perfect.
It was awesome.
Easy to understand and learn thank you for that
Thank you Madam for this wonderful website
I’m also a teacher of chemistry
that’s is good because our teacher tought us like this. than you madam
Thank you mam
It is easy to understand
This is very easy to understand all spectrum lines
Easy to understand contents and explain content ………….plausible
great explanation for all the topics
very useful
It is very useful for me
excellent to understand. thanks so much. i am equally a chemistry teacher
wonderful explanation,it has been challenging. iam a chemistry teacher