Contents
Salt of weak Acid and strong base
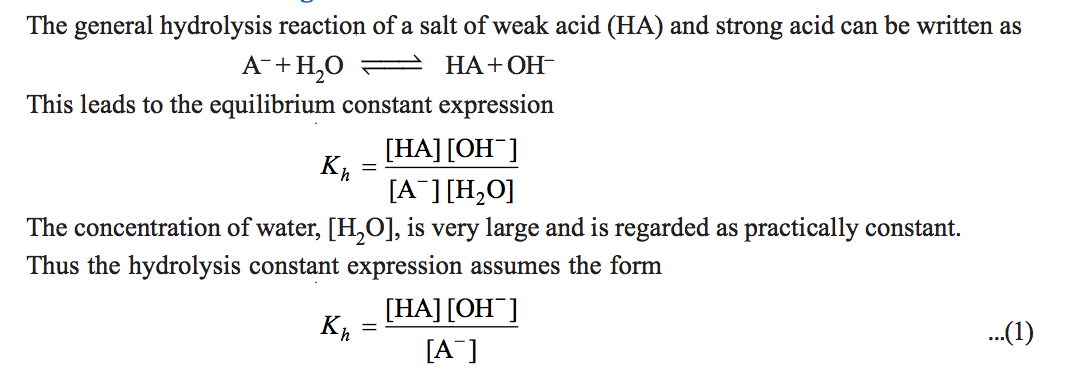
a) Hydrolysis Constant
b) Degree of Hydrolysis
A‾ + H2O OH‾ + HA
c 0 0 original molar conc
c(1-h ) ch ch Molar conc at equilibrium
c) pH
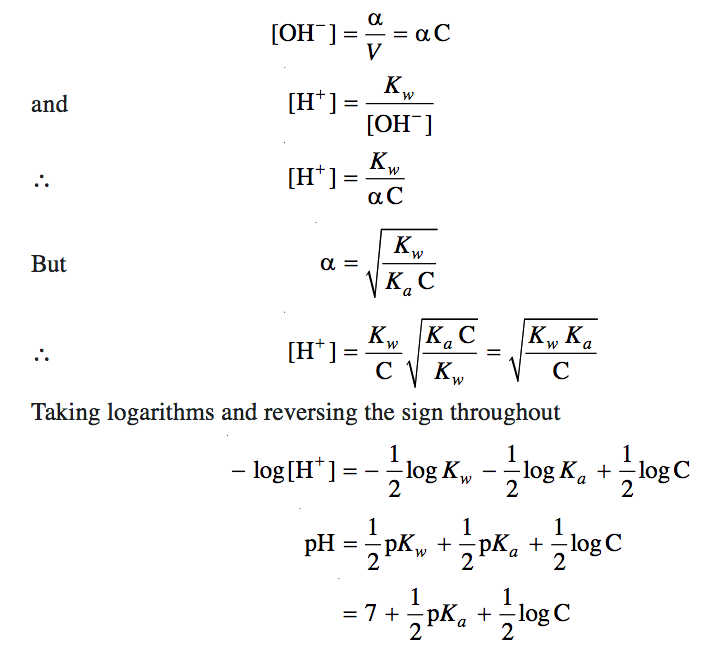
Salt of strong Acid and weak base
a) Hydrolysis Constant
BA + H2O BOH + HA
B+ + A‾ + H2O BOH + H+ + A‾
B+ + H2O BOH + H+
b) Degree of Hydrolysis
c) pH
B+ + H2O BOH + H+
c 0 0 original conc
c(1-h) ch ch conc at equilibrium
= ch = (Kw /Kb.c ) ½= (Kw .c /Kb)½ = (Kw .c /Kb )½
pH = – log
pH = -log (Kw .c /Kb )½
pH = -½ ( log Kw -log Kb + log c )
pH = ½ ( pKw -pKb – log c)
pH = 7 – ½(pKb + log c)
Salts of weak Acid and weak base
a) Hydrolysis constant
Kh = Kw / Ka .Kb
b) Degree of Hydrolysis
c) pH
HA H+ + A‾
= Ka (HA / A‾)
= Ka (ch / c(1-h))
= Ka (h /1-h)
√Kh = (h /1-h)
= Ka √Kh =Ka (Kw/ Ka .Kb)½ = (Ka .Kw /Kb)½
pH = -log
pH = – log (Ka .Kw /Kb)½
pH = -½( log Ka + log Kw -log Kb)
pH =½ (pKw +pKa -pKb)
pH = 7 + ½ (pKa – pKb)
If pKa < pKb , pH of the solution will be less than 7 and the solution will be acidic.
If pKa > pKb , pH of the solution will be greater than 7 and the solution will be basic.
If pKa = pKb , pH of the solution will be equal to 7 and the solution will be neutral.
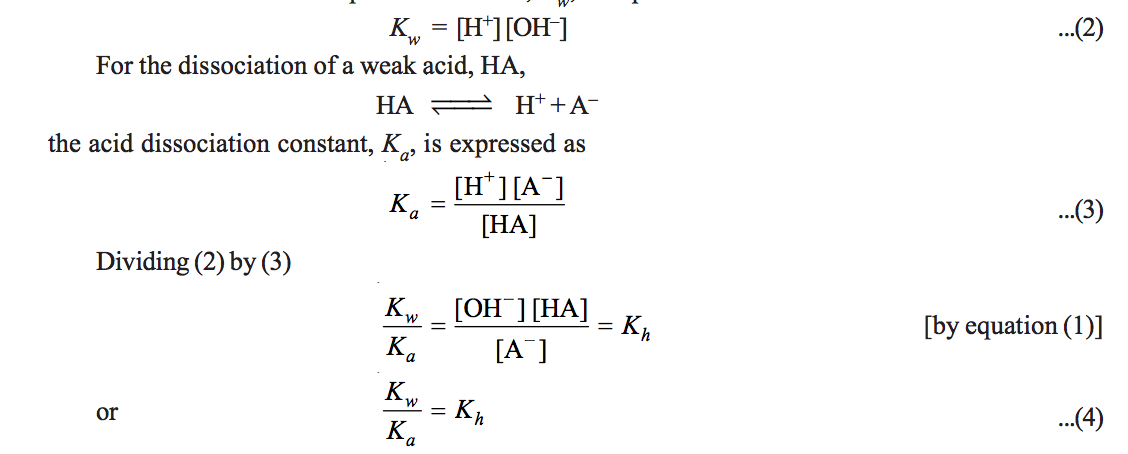
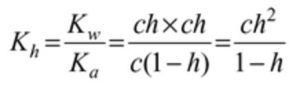
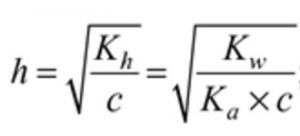
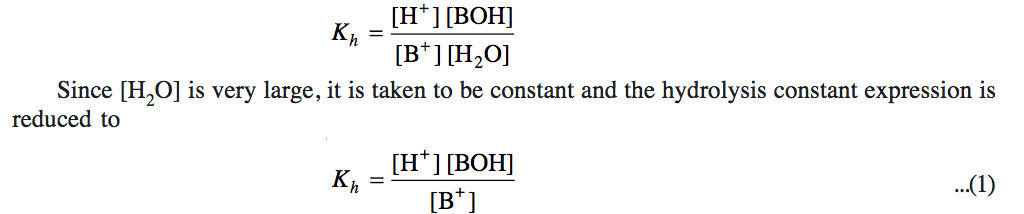
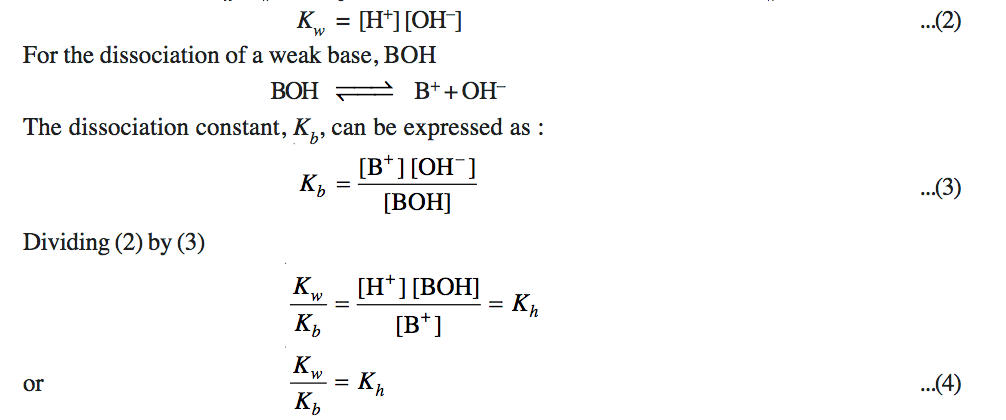

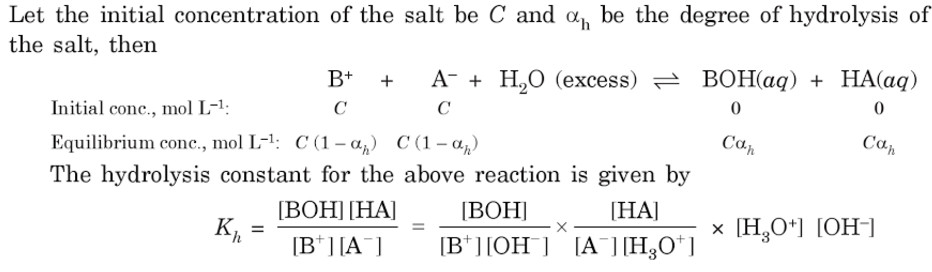
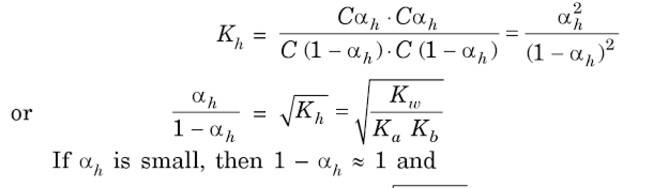
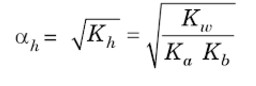
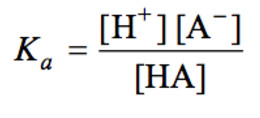
Nice, thanks a lot
10Q so much
I got the answer of my question
It’s awesome.
I have been searching for many days for these formulas.Thank u very much I will be following u from now onwards.
Good work sister
Thank you .
This is Awesome i really want this site in an app thank you
Thanks a lot………
Thank you so much maam !!!
I really liked that
Thanks
It’s great work. Thanks very much
I really want this thqnk you.
Thanks a lot mam
Thank you so much…
Thank you..good notes..
Thank you so much ,it helps me a lot For clearing my doubts