Hydrogen peroxide was discovered by the French Chemist J.L. Thenard in 1818.
Preparation
1)From sodium peroxide (Merck’s method)
Calculated amount of sodium peroxide is gradually added to an . ice-cold solution of 20% H2SO4.
Na2O2 + H2SO4 ——–> Na2SO4 +H2O2
Upon cooling crystals of Na2SO4·10 H2O separate out and the resulting solution contain about 30% H2O2.the solution also contains some dissolved Na2SO4 but it does not interfere with reactions of H2O2.
2) From Barium peroxide
a) By the action of dilute sulphuric acid: A thin paste of hydrated barium peroxide is prepared in ice cold water and then added slowly to an ice cold solution of 20% H2SO4 .
BaO2 · 8H2O (s) + H2SO4 (aq) ——> BaSO4(s) + H2O2(aq) + 8H2O(l)
The white precipitate of BaSO4 is removed by filtration leaving behind a dilute solution (5%) of H2O2.
Anhydrous barium peroxide cannot be used since the precipitated BaSO4 forms a protective layer around unreacted barium peroxide thereby preventing the further reaction.
limitation : Hydrogen peroxide prepared by this method contains appreciable quantities of Ba2+ ions which catalyse the decomposition of hydrogen peroxide. Therefore hydrogen peroxide prepared by this method cannot be stored for a long time.
H2SO4 also act as a catalyst for decomposition of hydrogen peroxide, therefore, the use of weaker acids such as H2CO3 and H3PO4 are preferred to H2SO4.
b) By the action of carbon dioxide or carbonic acid :
When a rapid stream of CO2 is bubbled through a thin paste of BaO2 in ice cold water, H2O2 and BaCO3 are produced.
BaO2 + H2O + CO2 ———> BaCO3 + H2O2
The insoluble barium carbonate is removed by filtration leaving behind dilute solution of hydrogen peroxide.
c) By the action of phosphoric acid :
Hydrogen peroxide can also be prepared by the action of phosphoric acid on barium peroxide.
3 BaO2 + H3PO4 ———> Ba3(PO4)2 ↓ + 3H2O2
Advantage : Since almost all the heavy metals impurities present in BaO2 and which catalyse the decomposition of hydrogen peroxide are removed as insoluble phosphates.As a result, the resulting solution of hydrogen peroxide has good keeping properties.
Manufacture of hydrogen peroxide
1) By electrolysis of 50% H2SO4 :
Hydrogen peroxide is manufactured by the electrolysis of a cold 50% solution of sulphuric acid at high current density in an electrolytic cell using platinum as anode and graphite as cathode.
2H2SO4 ——–> 2H+ + 2HSO4‾
cathode : 2H+ +2e‾ ——-> H2 ↑
anode : 2HSO4 ‾ ——–> H2S2O8 + 2e‾
Peroxodisulphuric acid formed around anode is withdrawn and then distilled with water under reduced pressure. The low boiling H2O2 distils over along with water leaving behind high boiling H2SO4 which is recovered and recycled.
H2S2O8 + H2O ——–> H2SO5 + H2SO4
H2SO5 + H2O ——-> H2SO4 + H2O2
If instead of 50% H2SO4, an equimolar mixture of H2SO4 and ammonium sulphate is electrolysed, a more concentrated solution of H2O2 is obtained.
(NH4)2SO4 + H2SO4 —-> 2 NH4HSO4
2 NH4HSO4 ——-> 2H+ + 2 NH4SO4‾
at cathode: 2H+ + 2e‾ ——–> H2 ↑
at anode 2NH4SO4‾ —–> (NH4)2S2O8 + 2e‾
Ammonium persulphate formed around anode is withdrawn and distilled with water to give hydrogen peroxide.
(NH4)2S2O8 + 2H2O —–> 2NH4HSO4 + H2O2
This process is now used for the laboratory preparation of D2O2 i.e.
K2S2O8 + 2 D2O ——> 2KDSO4 + D2O2
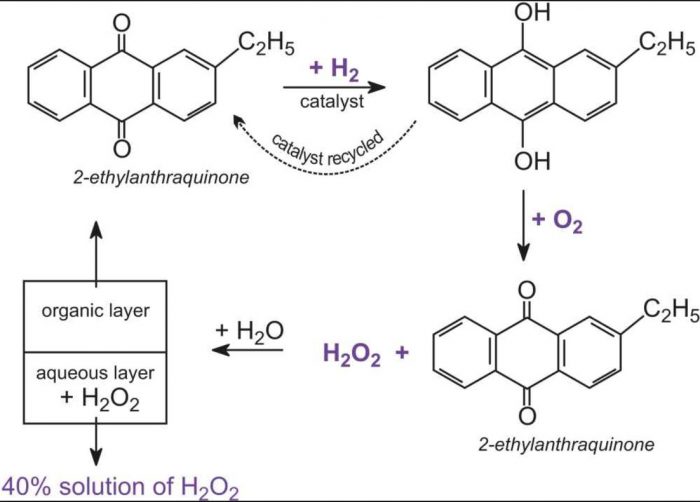
2) By autoxidation of 2-ethylanthraquinol
Air is bubbled through 10% solution of 2- ethylanthraquinol in benzene and cyclohexane when 2-ethylanthraquinol is oxidised to2- ethylanthraquinone and hydrogen peroxide.
Hydrogen peroxide thus formed is extracted with water and the aqueous solution is concentrated by distillation under reduced pressure to give 30% hydrogen peroxide solution.
2- Ethylanthraquinone formed in the process is reduced with hydrogen in the presence of Pd catalyst to give back 2- ethylanthraquinol which is again used.
Concentration of hydrogen peroxide solution
Hydrogen peroxide cannot be concentrated simply by distillation since its decomposers much below its boiling point to give water and oxygen.
Decomposition is catalysed by presence of heavy metal ion impurities, dust and rough surfaces.
Concentration of H2O2 is carried out carefully in a number of stages:
1) Evaporation on a water bath : The dilute aqueous solution of hydrogen peroxide is taken in a large shallow evaporating dish and is heated on a water bath. Slow evaporation of water continues until the solution contains about 50% hydrogen peroxide. Concentration of hydrogen peroxide by this method is not possible since it tends to decompose.
2) Dehydration in a vacuum desiccator
50% solution of hydrogen peroxide as obtained above is taken in a dish and place in a vacuum desiccator containing conc H2SO4 . Pressure inside the desiccator is reduced by connecting it to a vacuum pump. As a result of low pressure, water readily evaporates and the water vapour thus produced are absorbed by conc H2SO4.Hydrogen peroxide of about 90% concentration is produced.
3) Distillation under reduced pressure
The 90% solution of hydrogen peroxide as obtained above is subjected to distillation under reduced pressure. During this process, water distils over between 303-313 K leaving behind almost pure hydrogen peroxide.
4) Removal of last traces of water
The last traces of water in hydrogen peroxide are removed by freezing it in in a freezing mixture containing consisting of dry ice and ether when the crystal of hydrogen peroxide separates out. These crystals are removed, dried and melted to give pure hydrogen peroxide.
Storage of hydrogen peroxide
1) Hydrogen peroxide cannot be stored in glass bottles since the rough surface of glass, alkali oxides present in it, and exposure to light catalyse its decomposition. Therefore hydrogen peroxide is usually stored in coloured paraffin wax coated plastic or teflon bottles.
2) To prevent composition of hydrogen peroxide some stabilizer such as urea, glycerine, acetanilide, phosphoric acid must also be added.
Strength of hydrogen peroxide solution
The strength of an aqueous solution of hydrogen peroxide is usually expressed in following ways:
1) Percentage strength: It expresses the amount of hydrogen peroxide by weight present in 100 ml of the solution.
For Ex: 30% aqueous solution of hydrogen peroxide implies that 30 grams of hydrogen peroxide are present in 100mL of the solution.
2) Volume strength: The strength of an aqueous solution of hydrogen peroxide is in terms of the volume of oxygen liberated at NTP by the decomposition of 1 ml of that sample of hydrogen peroxide. The aqueous solution of hydrogen peroxide sold in the market are labelled as 10 volume, 20 volume, 30 volume, 100 volume. A solution of hydrogen peroxide labelled as 10 volume that 1 ml of such a solution of hydrogen peroxide on decomposition by heat produces 10 ml of oxygen at N.T.P.
Physical properties of Hydrogen Peroxide
1) Pure hydrogen peroxide is a thick syrupy liquid with pale blue colour.
2) It has a bitter taste.
3) Hydrogen peroxide is more dense and more viscous than water. This is due to the reason that the molecule of hydrogen peroxide are even more highly associated through hydrogen bonding then Water molecule.
4) Its melting point is to 272.4 K. Since it decomposes vigorously on heating, it is not possible to determine its boiling point at atmospheric pressure. Its boiling point has been determined to be 423.2 extrapolation method.
5) It is completely miscible with water, alcohol and ether in all proportion.
6)The dipole moment of hydrogen peroxide is little more than that of water.
Chemical properties
1) Decomposition
Pure hydrogen peroxide is an unstable liquid and decomposes into water and oxygen on long standing or heating.
2H2O2 (aq) —–> 2H2O(l) + O2 (g)
It is example of auto oxidation and auto reduction.
The decomposition is further accelerated by the presence of certain metal ions, metal powders and metal oxides.Even charcoal, rough surfaces and light also catalyse its decomposition. The solution of hydrogen peroxide must be handle with care since they may explode with traces of organic matter or even specs of dust.
2) Acidic nature
Pure hydrogen peroxide turns blue litmus red but its dilute solution is neutral to litmus. It thus behaves as a weak acid. Its dissociation constant is 1.55 × 10-12 at which at 298 K which is only slightly higher than that of water.
Hydrogen peroxide is only a slightly stronger acid than water. Since hydrogen peroxide has two ionizable hydrogen atom, it forms two series of salts i.e. hydroperoxides and peroxides.
H2O2 2H+ + HO2‾
H2O2 2H+ + O22‾
The acidic nature of hydrogen peroxide is shown by its neutralization reaction with Hydroxides.
NaOH + H2O2————> NaHO2 + H2O
2NaOH + H2O2————> Na2O2 + 2H2O
Since hydrogen peroxide is a weaker acid than carbonic acid therefore it does not decompose carbonates and bicarbonates to evolve carbon dioxide gas.
3) Oxidising and reducing character
Hydrogen peroxide behaves as an oxidising as well as reducing agent in both acidic and basic solution. The oxidation state of oxygen in hydrogen peroxide is -1 .It can be oxidised to oxygen or reduced to water or hydroxide ion.
Hydrogen peroxide is a powerful oxidising agent but a weak reducing agent.
Oxidising character
Hydrogen peroxide act as an oxidising agent both in acidic as well as alkaline medium .
In acidic medium
H2O2 (aq) + 2 H+ +2e‾ ———-> 2H2O (l)
In basic medium
H2O2 (aq) + 2e‾——-> 2OH‾ (aq)
Oxidising action in acidic medium
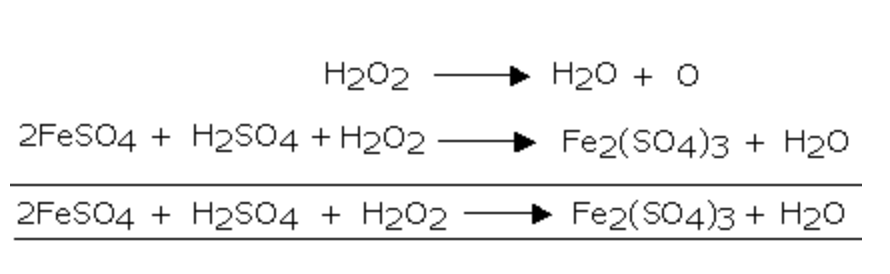
1) It oxidises ferrous sulphate to ferric sulphate
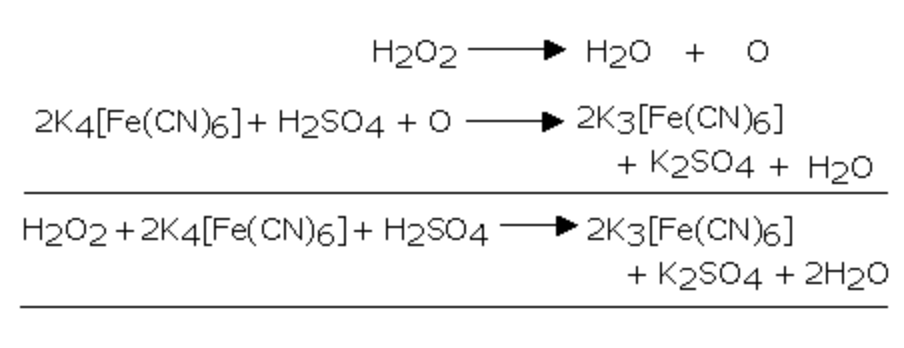
2) It oxidises potassium ferrocyanide to potassium ferricyanide
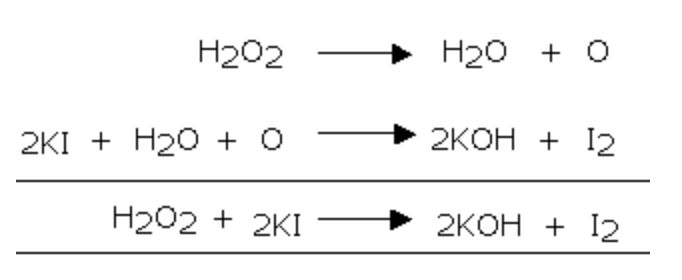
3) It liberates iodine from acidified potassium iodide solution
4) It reacts with ice cold acidified potassium dichromate solution to chromium pentoxide which dissolve in ether producing a blue colouration.
5) Hydrogen peroxide oxidises lead sulphide to lead sulphate
H2O2 ———-> H2O + [O] × 4
PbS + 4 [O] ———> PbSO4
_______________________________________________
PbS (s) + 4 H2O2 ————> PbSO4 + 4H2O
________________________________________________
This reaction is used in restoring the white colour of lead painting which have blackened due to the formation of lead sulphide by the action of H2S present in the air.On treatment with H2O2, lead sulphide changes into lead sulphate (white) and thus the colour of lead painting is restored.
Oxidising action in alkaline medium
1) It oxidises sulphites, nitrites and arsenites to sulphates, nitrates and arsenates in alkaline medium.
Na2SO3 + H2O2 ———-> Na2SO4 + H2O
KNO2 + H2O2 ———-> KNO3 + H2O
Na3AsO3 + H2O2 ———-> Na3AsO4 + H2O
2) It oxidises manganese salts to manganese dioxide
MnSO4 + 2NaOH + H2O2 ———-> Na2SO4 + MnO4 + 2H2O
3) It oxidises ferrous salts to ferric salts
2FeSO4 + 4NaOH + H2O2 ———->2 Fe(OH)3 + 2 Na2SO4
4)It oxidises Chromium salts to chromates
Cr2(SO4)3 +3H2O2 + 10 NaOH———-> 2 Na2CrO4 + 3 Na2SO4 + 8H2O
5) It oxidises formaldehyde to formic acid
HCHO + H2O2 ———-> HCOOH + H2O
Reducing character
In presence of strong oxidising agent, hydrogen peroxide behave as a reducing agent in acidic as well as alkaline medium.In these reactions, molecular oxygen is always produced by the combination of H2O2with the oxygen atom released by the strong oxidising agent.
H2O2 +[O] ——> H2O+ O2
Acidic medium
H2O2 (aq) —–> 2H+ + O2 + 2e‾
Alkaline medium
H2O2 (aq) + 2 OH¯ (aq) ———> 2 H2O(l) + O2 (g) + 2e‾
Reducing action in acidic medium
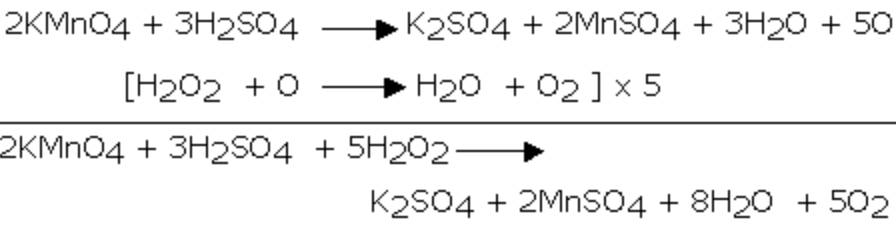
1) It reduces acidified potassium permanganate solution.As a result the pink colour of KMnO4 solution is discharged.
or
MnO4‾ + 8 H+ 5e‾ ——–> Mn2+ + 4 H2O ] × 2
H2O2 ——-> 2 H+ + O2 +2e¯ ]×5
__________________________________________________________
2 MnO4‾ + 6 H+ + 5 H2O2 ——-> 2Mn2+ + 8 H2O + 5O2
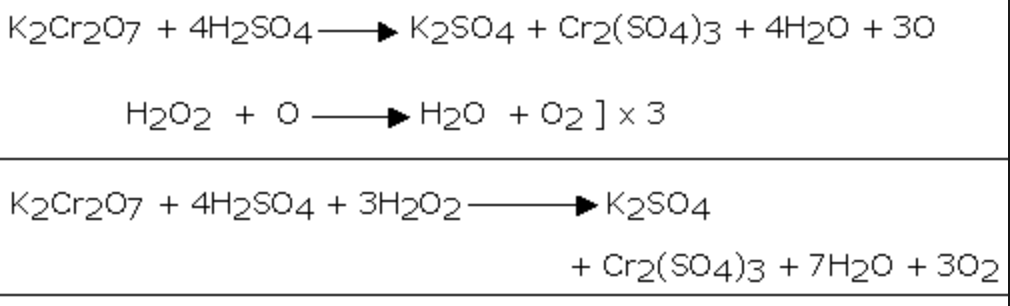
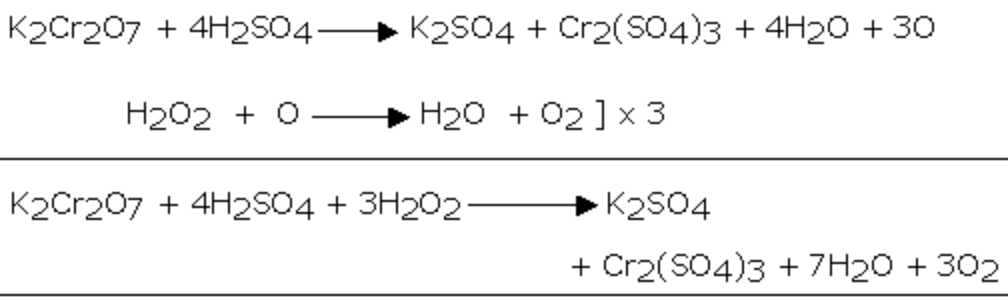
2) It reduces acidified potassium dichromate solution.As a result of this reaction, the orange colour of K2Cr2O7 changes to green due to the formation of chromium salt.
or
Cr2O72‾ + 14 H+ + 6e‾ ———–> 2 Cr3+ + 7H2O
H2O2 ——-> 2 H+ + O2 +2e¯ ]×3
_________________________________________________________
Cr2O72‾ + 8 H+ + 3 H2O2 ——-> 2 Cr3+ + 7H2O + 3O2
3) It reduces manganese dioxide to manganese sulphate in presence of dil H2SO4
MnO2 + H2SO4 ———-> MnSO4 + H2O + [O]
H2O2 + [O] —-> H2O + O2
___________________________________________________
MnO2 + H2SO4+ H2O2 ——>MnSO4 + H2O + O2
____________________________________________________-
4)It reduces ozone to dioxygen
O3 ——–> O2 + [O]
H2O2 + [O] —-> H2O + O2
_________________________________
H2O2 + O3 ——->H2O + O2
_________________________________-
5) It reduces chlorine to HCl
Cl2 + H2O ——-> 2 HCl + [O]
H2O2 + [O] —-> H2O + O2
______________________________
H2O2 + Cl2 —–> 2 HCl + O2
______________________________
Reduction in alkaline medium
1) It reduces potassium permanganate to manganese dioxide
2KMnO4 + 3 H2O2 ——-> MnO2 + 2KOH + 3 O2 + 2 H2O
2) It reduces ferric salts to ferrous salts
Fe2(SO4)3 + H2O2 + NaOH——–> 2 FeSO4 + Na2SO4 + O2 + 2 H2O
3) It reduces potassium ferricyanide to potassium ferrocyanide
2 K3 [Fe(CN)6] + 2 KOH + H2O2 ———> 2 K4[Fe(CN)6] + 2 H2O + O2
4) It reduces metal oxide to metals i.e. silver oxide to silver
Ag2O + H2O2 ———> 2 Ag + H2O + O2
5) It reduces halogen to halide ions
Cl2 + H2O2 + 2KOH——-> 2KCl + 2H2O + O2
I2 + H2O2 + 2NaOH——-> 2NaI + 2H2O + O2
6) It reduces hypohalites to halides
NaOBr + H2O2 —-> NaBr + H2O + O2
CaOCl2 + H2O2 —-> CaCl2 + H2O + O2
Bleaching Action
The bleaching action of hydrogen peroxide is due to the nascent oxygen which it liberates on decomposition.
H2O2 —–> H2O + [O]
The nascent oxygen combines with colouring matter which in turn gets oxidised.Thus the bleaching action of hydrogen peroxide is due to the oxidation of colouring matter by nascent oxygen.It is used for the bleaching of delicate material like ivory, feather etc.
Uses of hydrogen peroxide
1) The most important industrial use of hydrogen peroxide is as a bleaching agent for delicate materials like Silk, wool, paper pulp, straw, oils and fats.
2) It is used as a hair bleach and as a mild disinfectant.
3) It is extensively used to manufacture inorganic chemicals like sodium perborate and percarbonate which are important constituents of good quality detergents.
4) In the production of epoxides, propylene oxide and polyurethane.
5) Hydrogen peroxide is also used in the synthesis of hydroquinone, pharmaceuticals like cephalosporin and food products.
6) It is increasingly being used in Environmental chemistry to control pollution by treatment of domestic and industrial effluents, oxidation of cyanide and restoration of aerobic conditions to sewage waste.
7) It is used as an antiseptic under the name perhydrol for washing wounds, teeth and ears.
8) It is used for restoring the colour of lead painting which have blackened due to action of H2S present in the air on lead paints.
9) It is used in laboratory for detecting the presence of chromium, titanium and vanadium salts with which it yields peroxides of characteristic colours.
10) 93% hydrogen peroxide solution is used as an oxidant for rocket fuel and as a propellant.
11) It is used as antichlor in textile industry to remove excess of chlorine after bleaching operations.
12) A mixture of hydrazine hydrate and hydrogen peroxide with copper catalyst is used as a rocket propellant.
Test of hydrogen peroxide
1) Hydrogen peroxide on treatment with a acidified solution of Titanium salt gives a Yellow or Orange colour due to the formation of pertitanic acid.
Ti(SO4)2 + H2O2 + 2H2O ——> H2TiO4 + 2 H2SO4
2) It liberate iodine from KI solution which, in turn, gives blue colour with starch solution.
3) When an ethereal solution of H2O2 is shaken with acidified solution of K2Cr2O7 ,blue colour appears in the ethereal layer due to the formation of chromium pentoxide.
4) When brought in contact with hydrogen peroxide solution, a filter paper with black stain of PbS turns white.
5) It decolourizes acidified potassium permanganate solution.
Structure of hydrogen peroxide
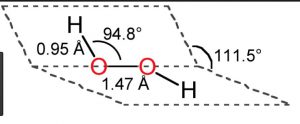
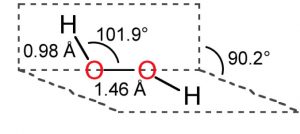
1) Hydrogen peroxide is a non – planar molecule.
2) The two oxygen atoms are linked to each other by a single covalent bond and each oxygen is further linked to a hydrogen atom by a single covalent bond.
3) The two O-H bonds are however in different planes due to repulsion between different bonding and antibonding orbitals.
4)The dihedral angle between the two planes being 111.5° in gas phase but it reduces to 90.2 ° in the crystalline state due to hydrogen bonding.
Dear Sir
Why Hydrogen per oxide gives yellow color with lime (calcium Hydroxide).Which impurity gives yellow color. please give me suggetion
With Regard
Thanks
Yours S.B.Trivedi