Contents
- 1 Electron gain Enthalpy
- 2 (1) Atomic Size
- 3 (2) Nuclear Charge
- 4 (3) Electronic Configuration
- 5 Variation along a period
- 6 Halogens have the most negative electron gain enthalpy
- 7 The electron gain enthalpy of fluorine is less negative than that of chlorine
- 8 The electron gain enthalpy of noble gases is positive
Electron gain Enthalpy
Electron gain enthalpy of an element may be defined as the energy released when a neutral isolated gaseous atom accepts an extra electron to form the gaseous negative Ion i.e. anion. It is denoted by
Δ eg H.
Greater the amount of energy released in the above process, higher is the electron gain enthalpy of the element. The electron gain enthalpy of a element is a measure of the firmness or strength with which an extra electron is bound to it.
Electron gain enthalpy is measured in electron volts per atom or kJ per mole.
The process of adding an electron to the atom can be either exothermic or endothermic.
Energy is released when an electron is added to the atom. Therefore, the electron gain enthalpy is negative.
The electron gain enthalpy for halogens is highly negative because they can acquire the nearest stable noble gas configuration by accepting an extra electron.
Noble gases have large positive electron gain enthalpy because the extra electron has to be placed in the next higher principal quantum energy levels there by producing highly unstable electronic configuration.
After the addition of 1 electron, the atoms becomes negatively charged and the second electron is to be added to a negatively charged Ion. But the addition of second electron is opposed by the electrostatic repulsion and hence the energy has to be supplied for the addition of second electron. The second electron gain enthalpy of an element is positive.
When an electron is added to oxygen atom to form O¯ ion ,energy is released. But when another electron is added to O¯ ion to form O2- ion, the energy is absorbed to overcome the strong electrostatic repulsion between the negatively charged O¯ ion and second electron being added.
Factors on which the electron gain enthalpy depends
(1) Atomic Size
As the size of the atom increases, the distance between the nucleus and the last shell which receives the incoming electrons increases. As a result, the force of attraction between the nucleus and the incoming electron decreases and hence the electron gain enthalpy becomes less negative.
(2) Nuclear Charge
As the nuclear charge increases ,the force of attraction between the nucleus and the incoming electron increases and hence the enthalpy becomes more negative.
(3) Electronic Configuration
Elements having exactly half filled or completely filled orbitals are very stable.
Energy has to be supplied to add an electron. Hence their electron gain enthalpy have large positive values since they do not accept the additional electrons so easily. The electron gain enthalpy becomes less negative in going from top to bottom in a group and more negative in going from left to right in a period.
Variation within a group
The electron gain enthalpy becomes less negative as we move down a group.
As we move down a group, both the atomic size and the nuclear charge increases. But the effect of increase in atomic size is much more pronounced then the nuclear charge. With increase in atomic size ,the attraction of the nucleus for the incoming electron decreases and hence the electron gain enthalpy becomes less negative.
The electron gain enthalpies of some of the elements of 2nd period i.e. O and F are less negative than the corresponding elements of the third period.
Reason: The elements of the second period have smallest atomic size among the elements in their respective group. As a result ,there are considerable electron electron repulsions within the atom itself and hence the additional electron is not accepted with the same ease as is the case with the remaining elements in the same group.
Chlorine has the most negative electron gain enthalpy.
Variation along a period
Electron gain enthalpy becomes more and more negative from left to right in a period.
As we move across a period from left to right the atomic size decreases and the nuclear charge increases. Both these factors tend to increase the attraction by the nucleus for the incoming electron and hence electron gain enthalpy becomes more and more negative in a period from left to right.
Halogens have the most negative electron gain enthalpy
The electron gain enthalpies of the halogen elements are the most negative. This is due to the reason that the valence Shell electronic configuration of the halogen is ns2 np5 and as such they require one more electron to acquire the stable noble gas configuration.
They have a strong tendency to accept an additional electron and hence the electron gain enthalpies are highly negative. As we move from chlorine to iodine, the electron gain enthalpies become less and less negative due to corresponding increase in their atomic radii.
In Cl, the additional electron enters the 3p subshell, in Br the additional electron enters the 4p subshell while in iodine it goes to the 5p subshell.
As the distance of the nucleus from the subshell which receives the additional electron increases, the force with which it is attracted by the nucleus decreases and hence the electron gain enthalpy become less negative as we move down the group from
Cl ————> Br ——–> I
The electron gain enthalpy of fluorine is less negative than that of chlorine
This is due to its small size. As a result of its small size ,the electron electron repulsion in the relatively compact to 2p subshell are comparatively large and hence the incoming electron is not accepted with the same ease as is the case with chlorine.
The electron gain enthalpy of noble gases is positive
The atoms of these elements have completely filled subshell. As a result there is no room in their valence orbitals and the additional electron has to be placed in an orbital of next higher shell. As a result, energy has to be supplied to add on additional electrons.
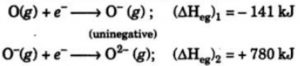
mast
Waoòoo… Very Well explained ….Very Good… Concept nicely Cleared….it will be more beneficial for me if such type of notes available for NEET exam.. Please mention the Website name and App so that I will be able to download and read it… 12 and 11 th Chemistry notes….
I Request you to make PDF of these notes so that we can download them to read offline.
I Request you to make the PDF’s of these notes so that we can read them offline
This is really helpful for studying Electron Gain Enthalpy thanks.
What a best explanation
Thank you for this beneficial notes
And the important thing is that any one can read this note for free.
Clearly understood
The best site for notes so far.
Thank you ma’am. You have no idea how many students are getting benefit out of this.
Good notes
It’s too benifificial , i am writing all these concept in my notes instead of writing my sir notes
I support the clarification about the topic itself-ionization of element. However, I wish you could add more examples
Superb ma’am… U explained the topic in a very simple manner