The first simple theory that was put forward to explain the shape of the molecule is known as Valence Shell Electron Pair Repulsion Theory.
This theory was given by Sidgwick and Powell in 1940.
1) The central atom is linked to other atoms by covalent bonds which are formed by sharing of electrons.
2) The central atom is surrounded by a shared pair of electrons and there may be some lone pairs of electrons also present.
3) If electron pairs surrounding the central atom are nearer, they will repel each other ,thereby increasing the energy of the molecules.
4) If the electron pairs are far apart, the repulsions between them will be less and so the energy of the molecule will be low.
The electron pairs surrounding the central atom repel one another and move so far apart from one another that there are no further repulsions between them. As a result, the molecule has minimum energy and maximum stability.
1) The shape of a molecule containing only two atoms is always linear.
2) For molecules containing three or more atoms, one of the atoms is called the central atom to which other atoms are linked.
3) If the central atom is linked to similar atoms and is surrounded by bond pairs of electrons only, the repulsions between them are similar as a result the shape of the molecule is symmetrical and the molecule is said to have regular geometry.
4) If the central atom is linked to different atoms or is surrounded by bond pair as well as lone pair of electrons, the repulsion between them are similar. As a result, shape of the molecule has an irregular or distorted geometry.
The order of repulsion between electron pairs as follow:
Lone Pair- lone pair > Lone Pair- bond- pair > Bond Pair- bond pair
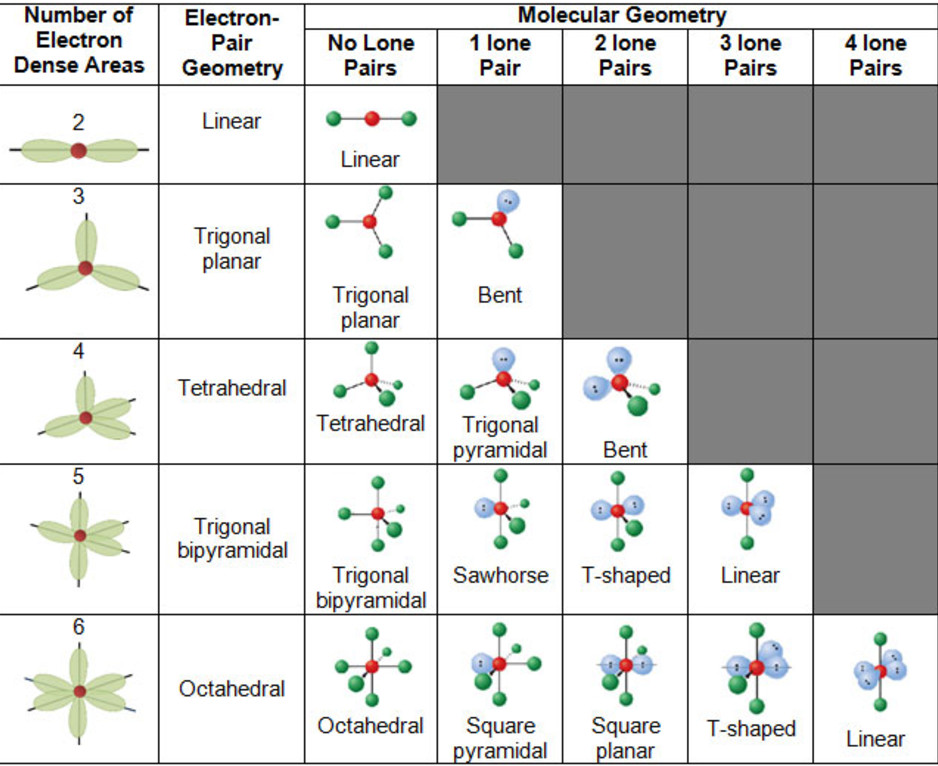
5) The exact shape of the molecule depend upon the total number of electron pairs present around the central atom.
Calculation of total number of electron pair, bond pair and lone pair and predicting the shape of the molecules and ions
1) Total number of electron pairs around the central atom = ½ (number of valence electrons of central atom + number of atoms linked to central atom by single bonds)
a) For negative ions ,add number of electrons equal to the units of negative charge on the ions to the valence electrons of the central atom.
b) For positive ions, subtract number of electrons equal to the units of positive charge on the ion from the valence electrons of the central atom.
2) Number of Bond pair= Total number of atoms linked to central atom by single bonds.
3) Number of lone pairs= Total number of electron- No of shared pair
Question On the basis of VSEPR theory, predict the shapes of BrF5 ?
Answer No. of valence electrons of the central Br atom = 7
No. of atoms linked to it by single bonds=5
Total no. of electron pairs around Br= 6
No. of bond pairs = No. of atoms linked to Br = 5
No. of lone pairs = 1
Therefore, the molecule is of the type AB5L
Hence, it has square Pyramidal shape.
nice explanation