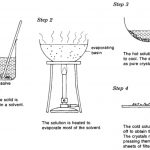
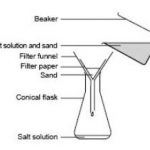
Here are some of the important methods which are commonly employed for the purification of organic compounds: Filtration The process of filtration is used to separate insoluble solid component of a mixture from the soluble components in a given solvent. It is used to separate a mixture of naphthalene and urea using water as solvent. Urea dissolves in water while … [Read more...] about Purification of Organic Compounds
sublimation
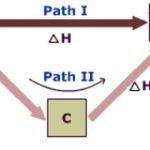
Enthalpy Changes During Phase Transitions
Enthalpy of Fusion Enthalpy of fusion is the enthalpy change accompanying the transformation of one mole of a solid substance into its liquid state at its melting point. it is also called molar enthalpy of fusion. The molar enthalpy of fusion ( Δ fus H ) of ice is +6 KJ mol-1. H2O ( s ) → H2O ( l ) Δfus H = + 6 KJ mol-1 The enthalpy of freezing has same value as … [Read more...] about Enthalpy Changes During Phase Transitions
Separation of Mixture
Question 1 What do you mean by separation of mixtures? Question 2 How will you separate a mixture containing sand and sugar? Question 3 Describe a method to separate a mixture of sand and salt? Question 4 How will you separate a mixture of salt and sugar? Question 5 Name a solvent used to separate a mixture of sulphur and carbon? Question 6 How will you separate … [Read more...] about Separation of Mixture
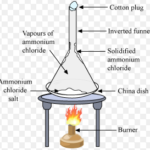
Sublimation
Question 1 What is sublimation. Question 2 Define sublimate. Question 3 Define sublime. Question 4 Give few examples of substances that undergoes sublimation ? Question 5 Why naphthalene balls kept in stored clothes in our homes disappear over a period of time ? Question 6 How does applying pressure help in the liquefaction of a gas ? Question 7 What is the … [Read more...] about Sublimation