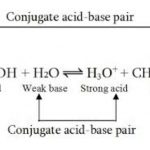
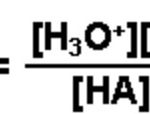Acids like HCl, H2SO4 ,HNO3 when dissolved in water dissociates almost completely thus producing a large number of H+ ions. These acids are called strong acids. Acids like CH3COOH , HF, H2CO3, H3PO4 dissociates only to a small extent in the aqueous solution giving small amount of H+ ions and hence are called weak acids. Bases like NaOH , KOH dissociate almost completely … [Read more...] about Dissociation or Ionization of Acids And Bases
strong base
Concepts of Acids and Bases
Classical concept of acids and bases Acid is a substance whose aqueous solution possessed the following characteristic properties 1) conduct electricity 2) reacts with active metals like zinc ,magnesium to give hydrogen 3) turns blue litmus to red 4) has a sour taste 5) whose acidic properties disappear on reaction with a base Base is a substance whose … [Read more...] about Concepts of Acids and Bases
Bases
Question 1 Define a base. Give example? Question 2 What are alkali? Question 3 What is common in all bases? Question 4 What are strong bases. Give example? Question 5 What are weak bases. Give example? Question 6 What happen when zinc granules are heated with sodium hydroxide solution? Question 7 What happen when a base react with non-metal … [Read more...] about Bases