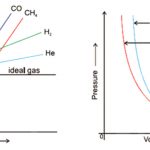
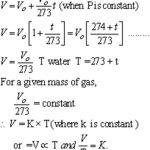A gas which obeys the ideal gas equation, PV = nRT under all conditions of temperature and pressure is called an ideal gas. There is no gas which obeys the ideal gas equation under all conditions of temperature and pressure. The gases are found to obey the gas laws if the pressure is low or the temperature is high. Such gases are known as real gases. It is found that … [Read more...] about Real Gases
real gas
Gas Laws
Boyle's law The relationship between the volume and pressure of a gas was studied experimentally by Robert Boyle in 1662. He used mercury and a simple U-tube. The pressure was increased by putting more mercury into the open limb. The volume of the air enclosed in the space above mercury in the shorter limb was noted each time. Boyle's law states that … [Read more...] about Gas Laws