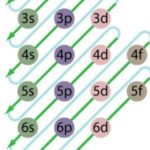
Energies of orbitals of hydrogen and hydrogen like particles depend upon the value of principal quantum (n) number only , those of multi-electron atoms depend both upon principal quantum number ( n ) as well as azimuthal quantum number(l). Diagram representing the arrangement of orbitals in order of their increasing energies are called energy level diagrams. Important … [Read more...] about Energy Level Diagram
Pauli exclusion principle
Pauli Exclusion Principle
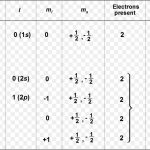
Wolfgang Pauli a German physicist in 1925 put forward a principle known after his name as Pauli exclusion principle. No two electrons in an atom can have the same set of four quantum number. In an atom, any two electrons may have the same values for any of the three quantum numbers but the 4th must be different. Any particular orbital is described by three quantum … [Read more...] about Pauli Exclusion Principle