Contents
- 1 Colligative Properties
- 2 Relative Lowering in Vapour Pressure
- 3 Relative lowering of vapour pressure-a colligative property
- 4 Determination of Molar Mass of a Solute from Relative Lowering in Vapour Pressure
- 5 Elevation in Boiling Point
- 6 Depression in freezing point- a colligative property
- 7 Determination of Molar mass of solute from Depression in Freezing point temperature
Colligative Properties
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called Colligative properties.
The four important colligative properties are:
(i) Relative lowering in vapour pressure
(ii) Elevation in boiling point
(iii) Depression in freezing point
(iv) Osmotic pressure.
Relative Lowering in Vapour Pressure
When a non-volatile solute is added to a solvent, the vapour pressure of the solution decreases.
Let xA be the mole fraction of the solvent, xB be the mole fraction of the solute and p be the vapour pressure of the pure solvent and p be the vapour pressure of solution.
Since solute in non-volatile, there will be no contribution of solute to the vapour pressure and the vapour pressure of the solution will be only due to the solvent. Therefore, the vapour pressure of the solution (p) will be equal to the vapour pressure of the solvent (pA), over the solution, i.e.,
p=pA
But, according to Raoult’s law, the vapour pressure of solvent is equal to the product of its vapour pressure in pure state and its mole fraction,
pA =p°A xA
pB = p°B xB
Since xA is always less than one, the vapour pressure of the solution is always less than pA° i.e., vapour pressure of the pure solvent.
The lowering in vapour pressure is
ΔpA = p°A – pA
ΔpA = p°A – p°A xA
xA = 1- xB
ΔpA = p°A – p°A(1- xB )
ΔpA = p°A – p°A + p°A xB
ΔpA = p°AxB
ΔpA / p°A = xB
p°A – pA / p°A = xB
where, pA – p°A (difference in vapour pressure of pure solvent and solution) represents the lowering in vapour pressure on the formation of solution and (p°A – pA )/p°A gives the relative lowering in vapour pressure. Thus, the relative lowering in vapour pressure of an ideal solution containing the non-volatile solute is equal to the mole fraction of the solute at a given temperature.
Relative lowering of vapour pressure-a colligative property
The relative lowering in vapour pressure depends only on the molar concentration of the solute (mole fraction) and is independent of its nature. Therefore, relative lowering in vapour pressure is a colligative property
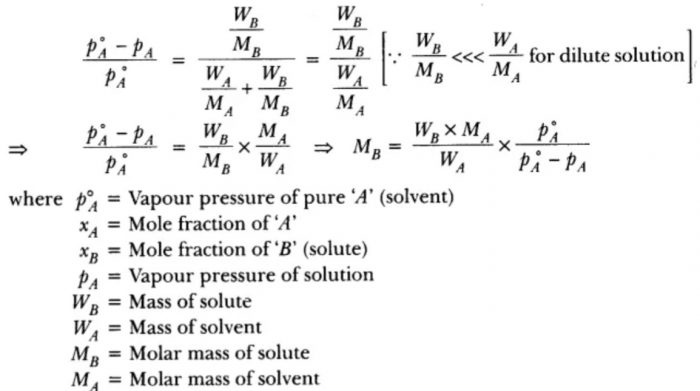
Determination of Molar Mass of a Solute from Relative Lowering in Vapour Pressure
A known mass of the solute is dissolved in a known quantity of solution and relative lowering in vapour pressure is measured experimentally.
According to Raoult’s law, the relative lowering in vapour pressure on the addition of a non-volatile solute to the solvent is:
(p°A – pA) / p°A = xB
Suppose wA and wB are the weights of the solvent and solute respectively and MA and MB are their corresponding molar mass. Then,
Mole fraction of solute = xB / nA+nB
where nA and nB are the moles of the solvent and the solute and
nA = wA /MA
Since the law is applicable to the ideal solutions which are dilute, the molar concentration of the solute being very small can be neglected as compared to that of the solvent. Thus,
Thus, the molar mass of the solute can be determined if the other quantities and relative lowering in vapour pressure are known.
Elevation in Boiling Point
The boiling point of a liquid is the temperature at which its vapour pressure becomes equal to the atmospheric pressure.
The vapour pressure of the solution containing non-volatile solute is less than that of the pure solvent. Therefore, the solution has to be heated to a higher temperature so that its vapour pressure becomes equal to the atmospheric pressure. Thus the boiling point of the solution is always higher than that of the pure solvent.
The difference in the boiling points of the solution (Tb) and pure solvent (Tb°) is called the elevation in boiling point (ΔTb).
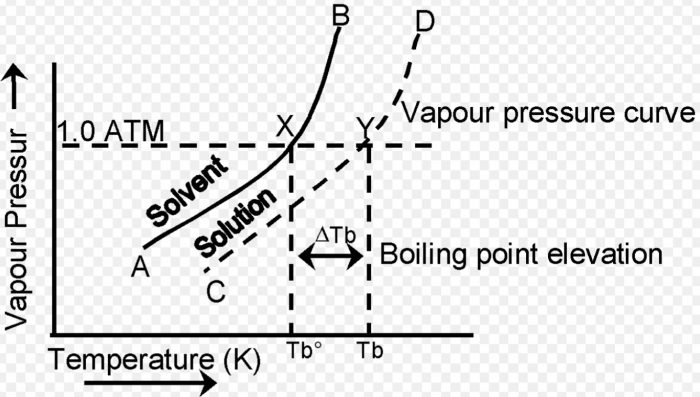
The vapour pressure of the pure solvent or solution increases with rise in temperature.
a) The curve AB gives the vapour pressure for the pure solvent and the curve CD gives the vapour pressure for the solution at different temperatures.
b) The curve CD representing the vapour pressure of the solution at different temperatures lies below the curve AB which corresponds to the vapour pressure of the pure solvent. This is because of the fact that the vapour pressure of the solution is less than that of the pure solvent at all temperatures.
c) The vapour pressure of the pure solvent becomes equal to atmospheric pressure at X (corresponding to temperature Tb°) while the vapour pressure of the solution becomes equal to atmospheric pressure at Y (corresponding to temperature Tb).
d) Thus, the b.p. of the pure solvent is Tb° while that of the solution is Tb .Since Tb is greater than Tb° there is an elevation or increase in boiling temperature of the solution as compared to that the solvent.
For example: Vapour pressure of water is 1.013 bar (or 1 atm) at 373 K. Therefore, water boils at 373 K because its vapour pressure at this temperature becomes equal to one atmospheric pressure which is 1.013 bar.The vapour pressure of an aqueous solution of sucrose is less than 1.013 bar at 373 K and therefore, the solution will not boil at 373 K. In order to make the solution to its temperature must be increased so that its vapour pressure becomes equal to 1.013 bar (1 atm).
Mathematically, elevation in boiling point, ΔTb , may be expressed as :
ΔTb = Tb – Tb°
The elevation in the boiling point (ΔTb) of a solution is proportional to the molal concentration of the solution, i.e.,
Tb ∝ m
ΔTb = Kb m
where m is the molality of the solution and represent moles of solute in 1 kg of solvent and Kb is called molal boiling point elevation constant or molal boiling point constant or ebullioscopic constant.
If m=1
then
ΔTb = Kb
Thus, molal boiling point elevation constant, Kb is defined as the elevation in boiling point for 1 molal solution i.e. a solution containing 1 gram mole of solute dissolved in 1000 g of the solvent.
Elevation in boiling point-a colligative property
Elevation in boiling point is directly proportional to the molal concentration of the solute (i.e., number of molecules) and therefore, it is a colligative property.
Determination of Molar Mass of Solute from Elevation in Boiling Point Temperature
The elevation in boiling point (ΔTb) is useful in determining the molar mass of the solute.
Let wB gram of a non-volatile solute is dissolved in wA grams of the solvent and MB is the molar mass of the solute.
Therefore, the molality, m of the solution is:
m = (Moles of solute x 100) / Wt. of solvent in grams
where moles of solute = wB /MB
m= (wB × 1000) / MB × wA
ΔTb = Kb m
ΔTb = (Kb × wB × 1000) / MB × wA
Thus , molar mass of solute
MB = (Kb × wB × 1000) / ΔTb × wA
Depression In Freezing point
At the freezing point of a solvent, the solid and the liquids are in equilibrium. This is only possible if they have the same vapour pressure.
Therefore, a solution will freeze when its vapour pressure becomes equal to the vapour pressure of the pure solid solvent.
Thus, the freezing point is the temperature at which the solid and the liquid states of the substance have the same vapour pressure. When a non-volatile solute is added to a solvent, the freezing point of the solution is always lower than that of the pure solvent.
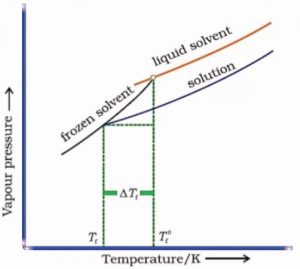
The orange curve gives vapour pressure of the pure solvent. The addition of a non-volatile solute lowers the vapour pressure and the purple curve gives the vapour pressure curve for the solution at different temperature.
The temperature corresponding to the point where the solid and liquid solvent meet (i.e., solid and liquid states have the same vapour pressure) represents the freezing point temperature of pure solvent (Tf°).
The temperature corresponding to the point where the solid solvent and liquid solution meet (i.e., solid and liquid states have the same vapour pressure) represents the freezing point temperature of the solution (Tf)
Since Tf, is less than Tf°, this shows that the freezing temperature of the solution is less than that of pure solvent and the depression in freezing temperature (ΔTf) is given as:
ΔTf = Tf°- Tf
The depression in freezing point of a solution is proportional to the molal concentration of the solution i.e.,
ΔTf ∝ m
ΔTf =Kf m
where Kf is the molal freezing point depression constant. It is also called molal cryoscopic constant.
If m=1
ΔTf =Kf
Thus, molal freezing point depression constant is defined as the depression in freezing point for 1 molal solution i.e, a solution containing 1 gram mole of solute dissolved in 1000 g of solvent.
Depression in freezing point- a colligative property
ΔTf ∝ m
The depression in freezing point temperature is directly proportional to the molal concentration of the solute (i.e., number of molecules) and therefore, it is a colligative property
Determination of Molar mass of solute from Depression in Freezing point temperature
The depression in freezing point temperature (ΔTf) is useful in determining the molar mass of the solute (MB).
Let wt. of the solute = wB g
Wt. of solvent = wA g
Molar mass of the solute = MB
Molality of solution, m = (wB × 1000) /(MB × wA)
By substituting the value of m in the relation , ΔTf = Kf × m
ΔTf =( Kf × wB × 1000 ) / MB × wA
MB = ( Kf × wB × 1000 ) / ΔTf × wA
super notes
Super easy and explanation is really very easy to understand
Really good….was helpful
I think, these must be the best notes in the world ……………..
Just in love with them ………………….
Really gud… It was so helpful for me
Thank you so much
I love this notes ……………………
….. this is the best notes