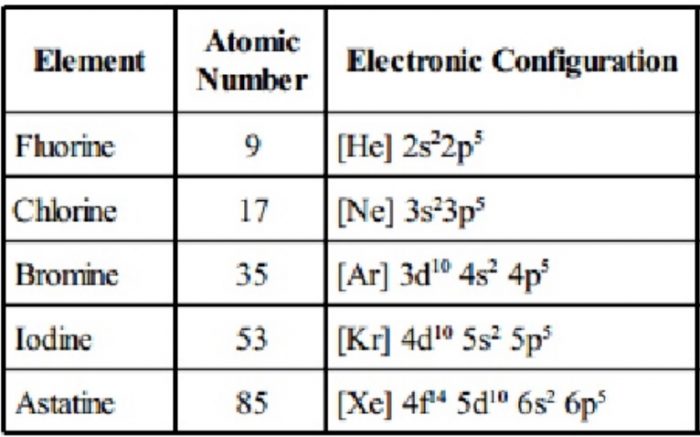
Group 17 Elements
Group 17 of the periodic table contains five elements : fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At). These are named as halogens. The name halogens is derived from two Greek words halo meaning sea salt and gens meaning born i.e., sea salt produce because the first three members occur as salts (chlorides, bromides and iodides) in sea water. These are among the most reactive non-metallic elements. The last member of the family, astatine is a radioactive element.
Occurrence
The halogens are very reactive and therefore, do not occur in the free state. However, all except astatine are abundant in the earth’s crust as halide ions, X¯.
Fluorine and chlorine are fairly abundant while bromine and iodine are comparatively less abundant. Fluorine is the thirteenth element in order of abundance in crystal rocks of the earth. It is present mainly as insoluble fluorides. The three most important minerals are
(i) fluorite : CaF2
(ii) cryolite : Na3AlF6 and
(iii) fluoroapatite : 3Ca3(PO4)2.CaF2 or Ca5(PO4)3F.
Physical Properties of Group 17 elements
(1) Electronic Configuration
The elements of this group have seven electrons in the outermost shell and have the general electronic configuration ns2np5.
(2) Atomic and ionic radii
The halogens have the smallest atomic radii in their respective periods due to maximum effective nuclear charge. The atomic and ionic radii increase with increase in atomic number. This is due to increase in the number of electron shells. The radius of the halide ion is always greater than the corresponding halogen atom. This is because the halide ion is formed by the gain of one electron by the atom. As a result, the number of electrons increases while the magnitude of nuclear charge remains the same. Therefore, the same nuclear charge acts on large number of electrons than are present in the neutral atom. Effective nuclear charge per electron is reduced and the electron cloud is held less tightly by the nucleus. This causes increase in size.
(3) Ionisation enthalpies
The ionisation enthalpies of halogens are very high. This indicates that they have very little tendency to lose electrons. However, on going down the group from fluorine to astatine, the ionisation enthalpy decreases. This is due to gradual increase in atomic size which is maximum for iodine.
(4) Melting and boiling points
The melting and boiling points of halogens increase with increase in atomic number as we go down the group.
Explanation: The forces existing between these molecules are weak van der Waal’s forces which increase down the group. At room temperature, fluorine and chlorine are gases, bromine is a liquid while iodine and astatine are solids.
(5) Electron Gain Enthalpies
(i) All these have maximum negative electron gain enthalpies in their respective periods. This is due to the fact that the atoms of these elements have only one electron less than the stable noble gas (ns2np6) configurations. Therefore, they have maximum tendency to accept an additional electron.
(ii) Electron gain enthalpy becomes less negative from top to bottom in a group. This is due to the fact that the effect of increase in atomic size is much more than the effect of increase in nuclear charge and thus, the additional electron feels less attraction by the large atom. Consequently, electron gain enthalpy decreases.
(iii) Fluorine has less negative electron gain enthalpy than chlorine. Therefore, chlorine has the highest negative electron gain enthalpy in this group. The less negative electron gain enthalpy of fluorine as compared to chlorine is due to very small size of the fluorine atom. As a result, there are strong inter electronic repulsions in the relatively small 2p subshell of fluorine and thus, the incoming electron does not feel much attraction. Therefore, its electron gain affinity is small.
Thus, negative electron gain enthalpy among halogens varies as :
F < Cl < Br
(6) Electronegativity
Halogens have large electronegativity values. The values decrease down the group from fluorine to iodine because the atomic size increases and the effective nuclear charge decreases. Fluorine is the most electronegative element in the periodic table.
(7) Metallic or non-metallic character
Because of very high ionisation energy values, all halogens are non-metallic in character. The non-metallic character decreases as we go down the group.
(8) Colour
All the halogens are coloured.
| Halogen | Fluorine | Chlorine | Bromine | Iodine |
| Colour | Light Yellow | Greenish Yellow | Reddish brown | Dark Violet |
Explanation
The colour of halogens is due to the fact that their molecules absorb radiations from visible light and the outer electrons are easily excited to higher energy levels. The amount of energy required for excitation depends upon the size of the atom. Fluorine atom is the smallest and the force of attraction between the nucleus and the outer electrons is very large. As a result, it requires large excitation energy and absorbs violet light (high energy) and therefore, appears pale yellow. Iodine needs very less excitation energy and absorbs yellow light of low energy. Thus it appears dark violet. Similarly, we can explain the greenish yellow colour of chlorine and reddish brown colour of iodine.
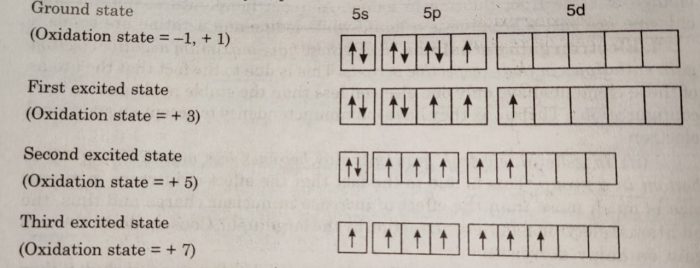
(9) Oxidation States
Halogens have only one electron less than the next noble gas. Therefore, they can get the noble gas configuration either by gaining one electron to form uninegative ion, X¯ or by sharing electrons with other atoms. Thus, they show an oxidation of state of -1 or + 1. Since fluorine is the most electronegative
element, it always shows an oxidation state of-1. The other elements also show positive oxidation states of +1, +3, +5 and +7. The higher oxidation states of chlorine, bromine and iodine are due to the presence of vacant d-orbitals in their valency shells. As a result the outer s or p electrons can easily be promoted to the vacant d-orbitals.
These higher oxidation states are realised mainly when the halogens are in combination with the small and highly electronegative fluorine and oxygen atoms. The oxidation states of +4 and +6 occur in oxides and oxoacids of chlorine and bromine and +7 oxidation state occurs in interhalogen compounds such as IF7.
Thus, the halogens exhibit the following oxidation states:
| Halogen | Oxidation state |
| Fluorine | -1 |
| Chlorine | -1, +1, +3 , +5, +7 |
| Bromine | -1 , +1, +3 ,+5 ,+7 |
| Iodine | -1, +1, +3, +5, +7 |
Its was very easy to understand and helpful for me .it clears my all doubt .thankqu for it