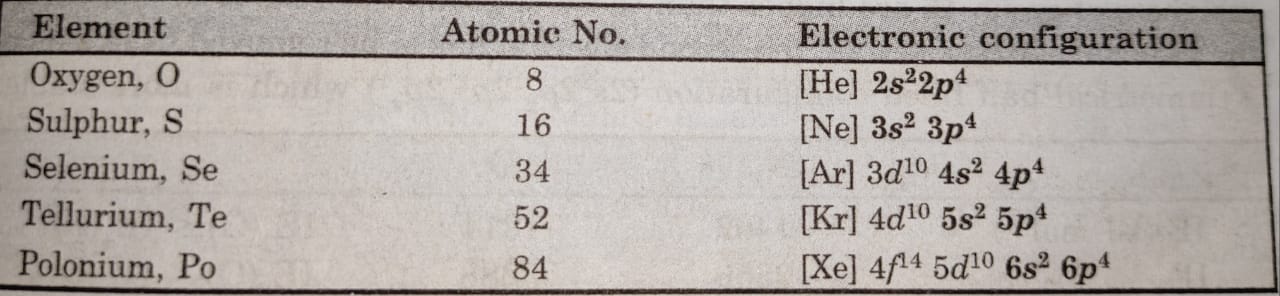
Contents
Chalcogens
The elements oxygen (O), sulphur (S), selenium (Se), tellurium (Te) and polonium (Po) constitute group 16 elements of the periodic table.
These are named as oxygen family after the name of the first member of the group. The first four elements of this group are collectively known as chalcogens (meaning ore forming elements) because many metal ores occur as oxides and sulphides.
Characteristics of Group 16 Elements
(1) Electronic Configurations
The elements of this group have six electrons in the outermost shell and have the general electronic configuration as ns²np4.
(2) Atomic and Physical Properties
(1) Atomic and ionic radii
The atomic and ionic radii of the elements of this group are smaller than those of the corresponding elements of group 15. The atomic and ionic radii of elements of group 16, as expected, increase on going down the group.
Reason: The smaller atomic and ionic radii of group 16 elements compared to group 15 elements are due to the increased effective nuclear charge of group 16 elements. As a result, there is greater attraction of the electrons by the nucleus and hence radii are less. The increase in the radii of group 16 elements down the group is due to the increase in the
number of electron shells.
(2) Ionisation enthalpies
The ionisation enthalpies of the elements of oxygen family are less than those of nitrogen family. As we move down the group from oxygen to polonium, the ionisation enthalpy decreases.
Reason: The ionisation enthalpy of oxygen should be more than that of N because of decrease in size. However, oxygen has unexpectedly low ionisation enthalpy than N. This is due to the reason that nitrogen has completely half filled orbitals and the configuration is stable because half filled and completely filled configurations have extra stability. But the configuration of O is less stable and therefore, has less ionisation enthalpy.
N(Z = 7) : 1s2 2s2 2px1 2py1 2pz1
(half filled, stable)
O(Z = 8) : 1s2 2s2 2px1 2py1 2pz1
(less stable)
The second ionisation enthalpies (IE2) of the members of group 16 are higher than those of group 15. This is because after the removal of first electron, the second electron has to be removed from a more symmetrical half filled configuration (2s2 2px1 2py1 2pz1) which is more stable.
As we move down a group there is increase in nuclear charge. But at the same time the atomic size as well as the number of inner electrons which shield the valence electrons from the nucleus increase. The overall effect of increase in atomic size and the shielding effect is much more than effect of increase in nuclear charge.
The outermost electron is less and less tightly held by the nucleus as we move down the group and hence ionisation energy decreases.
(3) Melting and Boiling Points
The melting and boiling points increase with the increase in atomic number as we go down the group.
Reason: When we move down the group, the molecular size increases. As a result, the magnitude of the van der Waals forces also increases with increase in atomic number and therefore melting point also increases. The melting point of polonium is, however, small.
(4) Electronegativity
The elements of group 16 have higher values of electronegativity than the corresponding elements of group 15. Oxygen is the second most electronegative element, the first being fluorine. The electronegativity decreases on going down the group. The decrease in electronegativity down the group is due to increase in size of the atoms.
(5) Metallic and non-metallic Character
The first four elements namely oxygen, sulphur, selenium and tellurium are non-metals. The non-metallic character is stronger in O and S are weaker in Se and Te.
(6) Electron gain Enthalpy
The elements of this family have high negative electron gain enthalpies. The values decrease down the group from sulphur to polonium. Oxygen, unexpectedly has low negative electron gain enthalpy.This is attributed to the small size of oxygen atom so that its electron cloud is distributed over a small region of space and therefore, it repels the incoming electron. Thus, the electron gain enthalpy of oxygen is unexpectedly less negative in the family.
(7) Catenation
Catenation is the tendency of an atom to form bonds with identical atoms. In this group, only sulphur has a strong tendency for catenation. Oxygen also shows this tendency to a limited extent. Thus, the polyoxides and polysulphides of the following types are known :
Polyoxides
H2O2, H-O-O–H
H2S2 , H-S-S–H
H2S3 H-S-S-S-H
H2S4 H-S-S-S-S-H
Polysulphides
(8) Elemental state
Oxygen exists as diatomic molecule. It exists as a gas. In oxygen molecule, there is pπ-pπ overlap between two oxygen atoms forming double bond, O=O. The intermolecular forces in oxygen are weak van der Waal’s forces and therefore, oxygen exists as a gas.
The other elements of family do not form stable Pπ-pπ bonds and do not exist as M, molecules. The other atoms are linked by single bonds and form polyatomic complex molecules.
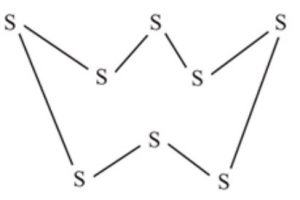
For example: Sulphur and selenium molecules have eight atoms per molecule (S8 and Se8) and have puckered ring structure.
(9) Allotropy
All the elements of the group exhibit allotropy.
For example : Dioxygen exists as O2 and O3 (ozone).
Sulphur exists in a number of allotropic forms of which yellow ortho-rhombic, α and β monoclinic forms are most important.
Selenium exists in eight allotropic forms of which three are red monoclinic forms containing
Se8 rings. The thermodynamically stable form is grey-hexagonal metallic selenium which consists of polymeric helical chains. The element exists as common amorphous black selenium. The grey selenium is the only allotrope of selenium which conducts electricity.
Tellurium has only one crystalline form having chain structure similar to that of grey selenium.
(3) Oxidation States
All the elements of this group have ns2 np4 configuration in their outermost shell. Therefore, the atoms of these elements try to gain or share two electrons to achieve noble gas configuration. Therefore, these elements show two types of oxidation states.
(i) Negative oxidation states: Oxygen has a very high value of electronegativity, it tends to achieve noble gas configuration preferably by gaining electrons. Thus, oxygen exhibits oxidation state of-2 in its compounds.
For example: The oxidation state of oxygen in OF2 is +2, while in H2O2 it is -1 and zero in O2 and O3. Since the electronegativites of these elements decrease as we move down the group, the tendency of these elements to show -2 oxidation state decreases down the group from S to Po.
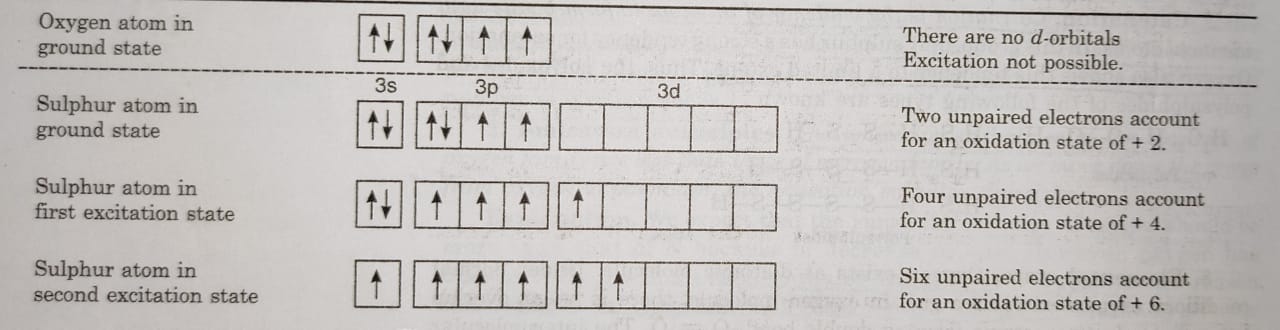
(ii) Positive oxidation states: Oxygen does not show positive oxidation states except in OF2. The other elements also show oxidation state of +2, +4 and +6 due to the promotion of electrons to vacant d-orbitals.
For example: In the ground state, sulphur has only two unpaired electrons and can form two bonds. This accounts for +2 oxidation state of S. If one of the paired electron of p-orbital is promoted to d-orbital, there are four unpaired electrons available for bonding. Therefore, S can show +4 oxidation state. On further excitation, the one of the 3s electron is promoted to vacant d-orbital and this makes available 6 unpaired electrons. This accounts for oxidation state of+6.
Compounds of S, Se and Te with O are typically tetravalent (+4 oxidation state). These +4 compounds show both oxidizing and reducing properties.
Fluorine brings about the maximum oxidation state of +6 in its compounds. Compounds in +6 oxidation state show only oxidising properties. The higher oxidation state becomes less stable in varying down the group. These compounds are covalent and hence are typically volatile.
excellent notes very precise for class 12…..thanks a lot mam
Help ful for students.Great thought by mrs.shilpi Nagpal.Thank you very much for this .