Contents
Chemical Properties of Group 16
Oxygen shows anomalous behaviour. This is due to its small size, high electronegativity, high ionisation enthalpy and absence of d-orbitals in its valence shell. Due to the absence of d-orbitals in oxygen, its covalency is limited to four.
The valence shell can be expanded using vacant d-orbitals and hence covalence exceeds four. Both oxygen and sulphur are very reactive and the reactivity decreases as O > S > Se > Te.
Despite of high bond dissociation enthalpy of O2 (493.4 kJ mol-1), the reaction of oxygen are highly exothermic. Once initiated, these reactions continue spontaneously (combustion) or even explosively. Sulphur is also very reactive element especially at high temperatures (which help the cleavage of S-S bonds). It combines with all elements except noble gases, N, Te, I, Pt and Au though their compounds with S are known. It also reacts with H2 slowly at 120°C and more rapidly at 200°C.
(1) Reactivity with hydrogen (formation of hydrides)
All the elements of group 16 form hydrides of the type H2E (where E = O, S, Se, Te, Po). For example: H2O, H2S, H2Se, H2Te and H2Po.
The hydrides of S, Se and Te are prepared by the action of acids on metal sulphides, selenides and tellurides respectively. H2S is prepared in the laboratory with the Kipps apparatus by the action of dilute sulphuric acid on FeS.
FeS(s) + 2H3O+(aq) → Fe2+ (aq) + H2S(g) + 2H2O(l)
Water is a colourless, odourless liquid while hydrides of the other elements of this group are colourless, bad smelling, poisonous gases.
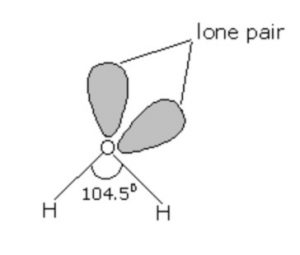
Structure of water
All these hydrides have angular structure which involves sp3 hybridisation of the central atom. For example: In water there are two bond pairs and two lone pairs. Due to the presence of lone pairs, the bond angle in water is less than the normal tetrahedral angle. It has been found to be 104.5°.
As we go down the group, the bond angle in the hydrides decreases as
| H2O | H2S | H2Se | H2Te |
| 104.5° | 92.1° | 91° | 90° |
Explanation
As we move down the group from O to Te, the size of the central atom goes on increasing and its electronegativity goes on decreasing. The position of bond pairs of electrons shifts more and more away from the central atom in moving from H2O to H2Te.
For example: The bond pair in O-H bond is closer to oxygen than the bond pair in S-H bond. As a result, the force of repulsion between the bonded pairs of electrons in H2O is more than in H2S. In general, the force of repulsion between the bonded pairs of electrons decreases as we move from H2O to H2Te and therefore, the bond angle decreases in the same order as : H2O > H2S > H2Se > H2Te
Trends in characteristics :
(i) Volatility : All the hydrides are volatile. The volatility increases from H2O to H2S and then decreases.
| H2O | H2S | H2Se | H2Te |
| 373 K | 213 K | 232 K | 269 K |
Thus, the trend of volatility is H2O < H2S < H2Se < H2Te
Explanation: The high boiling point and therefore low volatility of water is due to the association of H2O molecules through hydrogen bonding. However, hydrogen bonding is not present in other hydrides. The intermolecular forces between the hydrides (except H2O) are van der Waal’s forces. These forces increase with increase in molecular size and therefore, boiling points increase on moving from H2S to H2Se.
(ii) Acidic character
The hydrides of this group are weakly acidic in character.
For example: H2S behaves as weak diprotic acid ionising as :
H2S ⇔ H+ + HS¯
HS¯ ⇔ H+ + S2-
The acidic character increases from H2O to H2Te as : H2O < H2S < H2Se < H2Te
Explanation:
The decreasing acidic strength of the hydride is evident from their dissociation constant (K) values.
| Hydrides | H2O | H2S | H2Se | H2Te |
| Ka | 1 × 10-14 | 1.3 × 10-7 | 1.3 × 10-4 | 2.3 × 10-2 |
The increase in acidic character from H2S to H2Te can be explained on the basis of size of the central atom. As the size of the central atom increases in the order O<S< Se < Te, the distance between the central atom and hydrogen also increases. As a result of increase in bond length, the bond dissociation enthalpy decreases and bond cleavage becomes more and more easy. Therefore, the acidic strength of the hydrides increases down the group.
(iii) Thermal stability
The thermal stability of the hydrides decreases
from H2O to H2Te as : H2O > H2S > H2Se > H2Te
Explanation
On going down the group, the size of the central atom increases and therefore, its tendency to form stable covalent bond with hydrogen decreases. As a result, the M-H bond strength decreases and therefore, thermal stability decreases.
(iv) Reducing character
All the hydrides except water are reducing agents. The reducing power of these hydrides increases from H2O to H2Te.
H2O < H2S < H2Se < H2Te
This is due to the decrease in thermal stability of the hydrides. Greater the unstability of hybride, the greater is its reducing character.
Oxygen and to a greater extent sulphur differ from the remaining elements in their tendency to form polyoxides and polysulphides, which are less stable than normal salts.
The two common examples are H2O2 and H2S2
H2O2 is fairly stable whereas H2S2 is unstable and decomposes readily to give sulphur and hydrogen sulphide. The decomposition is accelerated by the presence of hydroxyl ions.
H2S2 → H2S + S
H2O2 is a strong oxidising agent but H2S2 is not.H2O2 form highly associated molecule because of hydrogen bonding while H2S2 forms discrete molecules.
Sulphur forms a few hydrogen polysulphides called sulphanes. Some of these are :
H2S2 Hydrogen disulphide H-S-S-H
H2S3 Hydrogen trisulphide H-S-S-S-H
H2S5 Hydrogen pentasulphide H-S-S-S-S-S-H
(2) Reactivity with Oxygen (formation of oxides)
All the elements of group 16 form two main oxides EO2 and EO3 (E = S, Se, Te or Po).
Oxides of sulphur are more stable than the corresponding oxides of other elements.
For example: SO2 and SO3 are more stable than the corresponding dioxides and trioxides.
(i) Monoxides
All elements except selenium form monoxides. Sulphur monoxide is formed by heating a mixture of sulphur dioxide and sulphur under reduced pressure at 425-475 K.
SO2 + S → 2SO
It can also be prepared by the action of silver on thionyl chloride
SOCl2 + 2Ag → SO + 2AgCl
Tellurium monoxide and polonium monoxides are formed by heating their corresponding trioxides. Monoxides are assigned the structure E=O (E = S, Te, Po).
(ii) Dioxides
All the members of oxygen family (S, Se, Te and Po) form dioxides of the formula EO2 (e.g. , SO2, SeO2, TeO2, PoO2). The dioxides are obtained by direct union of elements :
E(s) + O2(g) → EO2(E)
S8 (s) + 8 O2 (g) → 8 SO2 (g)
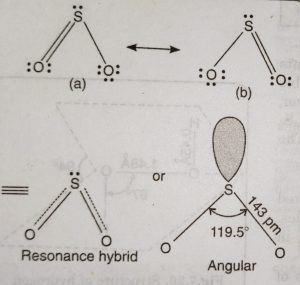
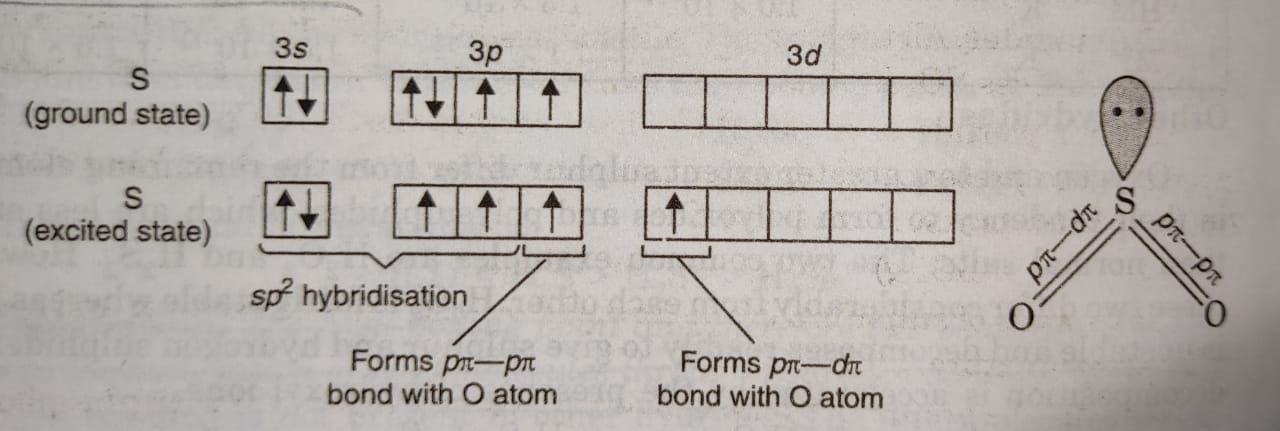
The dioxides of these elements differ considerably in their properties and structure. SO2 is a gas at room temperature (b.p. = 263 K). In SO2, S atom undergoes sp2 hybridisation.
Two of the three sp2 hybrid orbitals form two σ-bonds with oxygen atoms. The remaining two unhybridised orbitals (3p and 3d) form pπ-pπ and pπ-dπ double bonds with O atom. Thus, SO2 has bent or angular structure.
The third sp2 hybrid orbital is occupied by a lone pair of electrons. Since one of the orbital is occupied by a lone pair of electrons and the repulsion between lone pair-bond pair is more than between bond pair- bond pair, the bond angle of OSO is slightly reduced from 120° to 119.5°.
As is clear, the two π bonds are different because one is formed by pπ-pπ overlap and the other is formed by pπ-dπ overlap. But actually both the S-O bonds have exactly same bond length (143 pm). This is because of resonance between two structures. The SO2 molecule is resonance hybrid of two structures (a) and (b).
The multiple bonding in S-O bond in SO2 is due to pπ-pπ or pπ-dπ bonding.There is possibility of pπ-dπ bonding due to overlap of filled pπ orbitals of oxygen with vacant 3d-orbitals of sulphur.
SeO2 is a white crystalline solid. In the gaseous state, it exists as discrete molecules having structure similar to SO2 (a). In the solid state, it has a polymeric structure consisting of infinite chains (b).
TeO2 and PoO2 are also non-volatile crystalline ionic solids and occur in two crystalline forms each. The dioxides of elements of group 16 have different structures because the tendency of these elements to form pπ-pπ multiple bonds decreases as the atomic size increases down the group.
Properties
(1) SO2 dissolves giving H2SO3 which exists only in solution and cannot be isolated.
SO2 + H2O → H2SO3
The gas is, therefore, known as sulphurous anhydride. Therefore, the aqueous solution is acidic turning blue litmus to red, Ir the solution is boiled, all the sulphur dioxide may be removed.
H2SO3 → H2O + SO2 ↑
SeO2 dissolves in water giving selenious acid, H2SeO3 which can be isolated in a crystalline state. TeO2 is almost insoluble in water. However, TeO2 dissolves in alkalies to form tellurites and in acids to form basic salts showing that it behaves as amphoteric.
(iii) Trioxides
All the elements of this group form trioxides of the formula EO3.
Amongst trioxides SO3 is most important, while SeO3 and TeO3 are also known.
Sulphur trioxide is prepared by direct combination of sulphur dioxide and Oxygen in the presence of finely divided platinum or divanadium pentoxide at 2 atmospheric pressure and 450°C.
SO2(g) + O2(g) → 2SO2(g)
The vapours of sulphur trioxide formed are collected in a receiver and cooled by freezing mixture.
a) It can also be prepared by the dehydration of sulphuric acid with phosphorus pentoxide.
H2SO4 → SO3 + H2O
b) Sulphur trioxide can also be prepared by heating ferric sulphate, which decomposes to give sulphur trioxide.
Fe2(SO4)3 → Fe2O3 + 3 SO3
Structure
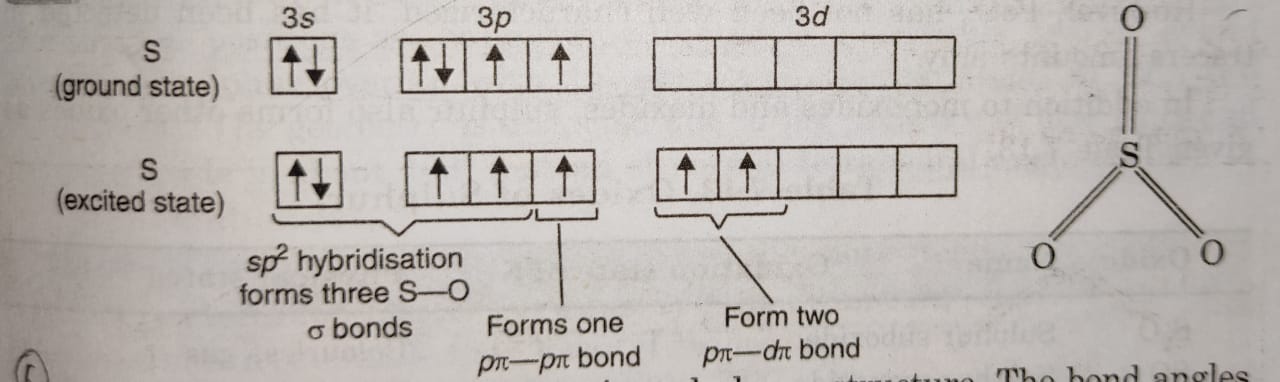
(1) SO3 has trigonal planar structure in the gaseous state in which S atom is sp2 hybridised.
(2) The three sp2 hybrid orbitals of S overlap with p-orbitals of O to form three S-O sigma bonds.
(3) The sulphur atom in the excited state is now left with one p and two d-orbitals which overlap with p-orbitals of O to form three π-bonds.
(4) In this one p-orbital of O form pπ-pπ bond with p-orbital of S while other two O atoms overlap with d-orbitals of S atom forming pπ-dπ bonds.
(5) Therefore, SO3 molecule has a trigonal planar structure. The bond angles in SO3 are exactly 120° each and S=0 bond lengths are 142 pm.
(6) The three S-O π bonds are different (one pπ-pπ and two pπ-dπ) yet they are equal because of resonance.
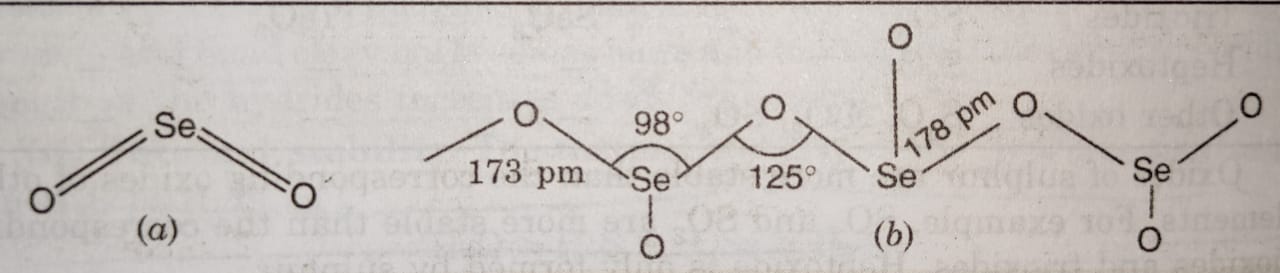
(7) The molecules of SO3 are held together by weak van der Waal’s forces of attraction. Therefore, it exists as a gas at room temperature. However, in the solid state, it exists as linear polymeric chain structure or cyclic trimer.
SeO3 is prepared by the direct oxidation of Se or SeO2. It is white hygroscopic solid having melting point 118°C. It exists as monomer in the vapour state and as a cyclic tetramer (Se4O12) in the crystalline state.
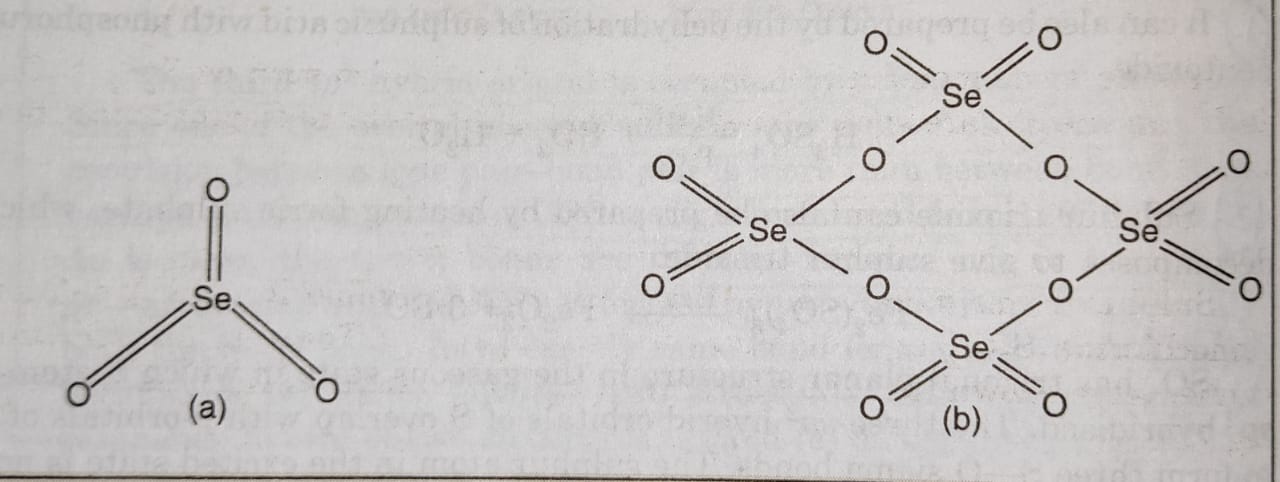
(3) Reactivity with halogens (formation of halides)
The elements of group 16 form a large number of halides of the type EX6 ,EX4 and EX2 (X=halogen).
The stability of the halides decrease in the order
F¯> Cl¯> Br¯ > I¯
Since fluorine is more electronegative than oxygen, its compounds with oxygen are called fluorides. For example : F2O should be written as OF2 and is named as oxygen difluoride. But since oxygen is more electronegative than Cl, Br and I the compounds of these halogens are called oxides.
(i) Monohalides
The stability of the halides decreases in the order:
F> Cl > Br > I
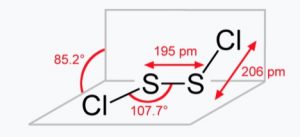
Amongst the monohalides, the dimeric monohalides such as S2F2, S2Cl2, Se2Cl2 and Se2Br2 are known. These dimeric halides undergo disproportionation as :
Se2Cl2 → SeCl4 + 3Se
Sulphur forms monohalides such as sulphur monofluoride (S2F2), sulphur monochloride (S2Cl2) and sulphur monobromide (S2Br2).
(ii) Dihalides
All the elements except selenium form stable dichlorides and dibromides.
Amongst the dihalides, SCl2 is best known. SCl2 is angular in shape. In this case S atom undergoes sp3 hybridisation, in which two positions are occupied by lone pairs of electrons. Two sp3 hybrid orbitals of sulphur overlap with 3p-orbitals of chlorine atoms to form two S-Cl bonds. The geometry is distorted due to the presence of lone pairs and the bond angle is about 103° instead of normal tetrahedral angle of 109.5°.
(iii) Tetrahalides
Amongst tetrahalides, tetrafluorides are the most stable.
Amongst tetrafluorides, SF4 is a gas, SeF4 liquid and TeF4 solid.
SF4 is highly reactive and is most stable than lower fluorides.
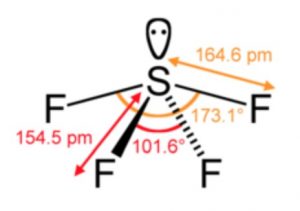
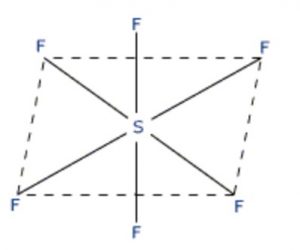
These tetrafluorides (e.g SF4) involve sp3d hybridisation and have trigonal bipyramidal structure in which one of the equatorial positions is occupied by a lone pair of electrons. This geometry is regarded as see-saw geometry. Due to larger lone pair-bond pair repulsion than bond pair-hond pair repulsion, the bond angle decreases from 180° to 173°.
(iv) Pentahalides
Only sulphur forms one pentahalide, S2F10. In this two SF5 units are linked through sulphur atoms having octahedral geometry.
(v) Hexahalides
Among hexahalides, only hexa fluorides are stable compounds.These are all gaseous in nature. SF6 is extremely stable and chemically inert for stearic reasons. In SF6 the six F atoms protect the sulphur atom from attack by reagents to such an extent that even thermodynamically most favourable reactions like hydrolysis do not occur. In contrast, the less stearically hindered SF4 undergoes hydrolysis readily.
SF4 + 2H2O → 4HF + SO2
SF6 + H2O → No reaction
SeF6 is slightly more reactive and TeF6 is still more reactive. TeF6 is rapidly hydrolysed with water.
TeF6 + 6H2O → H2TeO6 + 6HF
SF6 has an octahedral structure in which sulphur undergoes sp3d2 hybridisation.
Very well explained Ma’am.