Contents
- 1 Extraction of Crude Metal from Concentrated Ore
- 2 Conversion of the concentrated ore to its oxide form (Oxidation or de-electronation)
- 3 (B) Conversion of the oxide of the metal to the metallic form (Reduction or electronation)
- 4 (1) Reduction by precipitation (Hydro-metallurgy) or Displacement method
- 5 (2) Electrolytic Reduction
Extraction of Crude Metal from Concentrated Ore
The process of working of the concentrated ore to extract metal depends upon the nature of the ore as well as the nature of the impurities present in the ore.
The concentrated ore must be converted into a form which is suitable for reduction. Generally, the sulphide ores are converted to oxides before reduction because oxides are easily reduced.
The main operations for the working of the ore involves two steps:
(1) Conversion of the concentrated ore to its oxide form (oxidation or de-electronation)
(2) Conversion of the oxide to the metal (reduction or electronation)
Conversion of the concentrated ore to its oxide form (Oxidation or de-electronation)
The concentrated ore is either a hydrated oxide, a carbonate or a sulphide. It can be converted into its oxide form by the following two methods :
(i) Calcination
(ii) Roasting
(i) Calcination
Calcination is a process of heating the ore strongly either in a limited supply of air or in the absence of air. During calcination, the following changes take place :
(i) moisture is removed
(ii) the volatile impurities are removed
(iii) the ore becomes porous
(iv) water from hydrated oxides is removed
(v) carbonates decompose to oxides.
For example
(1) Zinc occurs as zinc carbonate in calamine (ZnCO3). The ore is calcined i.e., heated strongly in the absence of air to convert it to zinc oxide. During calcination, carbon dioxide is expelled.
ZnCO3 (s) ———-> ZnO (s) + CO2 (g)
Zinc Carbonate
(Calamine ore)
CaCO3(s) ——> CaO(s) + CO2(g)
CaCO3 .MgCO3 (s) ———> CaO(s) + MgO(s) + 2 CO2(g)
(2) Aluminium occurs as Al2O3.H2O in its bauxite ore. When the bauxite ore is calcined, water vapours are expelled and anhydrous aluminium oxide is obtained.
Al2O3.H2O —–> Al2O3 + 2H2O
(3) Water of hydration are removed from limonite (Fe2O3.3H2O).
Fe2O3.3H2O —–> 2 Fe2O3 + 3 H2O
(ii) Roasting
Roasting is the process of heating the ore strongly in the presence of excess of air at a temperature below the melting point of the metal.
As a result of roasting, the moisture and volatile impurities are removed and the ore is converted to oxide.
For example: Impurities of sulphur, arsenic and phosphorus are, removed as their volatile oxide, SO2 , As2O3 , P2O5.
S + O2 —-> SO2
4 As + 3 O2 —-> 2 As2O3
P4 + 5 O2 —-> 2 P2O5
The ores of the metal are converted into their oxides. For example, the sulphide ore of the metal is roasted to give oxide.
2ZnS + 3O2 ——–> 2ZnO + 2 SO2
2PbS + 3 O2 ——–> 2PbO + 2 SO2
2 Cu2S + 3 O2 ——–> 2Cu2O + 2 SO2
The SO2, produced is utilised for the manufacture of H2SO4
Sometimes, the oxidation of sulphides takes place only to the sulphate stage.
For example
PbS + 2 O2 —-> PbSO4
ZnS + 2 O2 —> ZnSO4
Both calcination and roasting are generally carried in a reverberatory furnace. In case of roasting, the air holes are kept open while in case of calcination, the air holes are partially or completely closed.
(B) Conversion of the oxide of the metal to the metallic form (Reduction or electronation)
The metal oxides are usually reduced to free metals by using a suitable reducing agent such as carbon, carbon monoxide or even another metal.
The process of extraction of metal by heating the metal oxide with a suitable reducing agent is called thermal reduction or pyrometallurgy. Some metals are easily reduced (i.e., reduction occurs at low temperatures) while others are reduced with difficulty (i.e. reduction occurs at high temperatures).
Depending upon the nature of the oxide and metal, the extraction of metal can be carried out by the following reducing agents:
(i) Reduction with C or CO: In the metallurgy of Fe, Cu, Pb, Sn, Zn, Mg, Co, etc.
(ii) Reduction with Na, Al, Mg or hydrogen : In the metallurgy of Mn, Cr, Ti, Mo, W, etc.
(iii) Reduction with water gas (CO, H2): In the metallurgy of Ni.
(iv) Self reduction or Auto-reduction : In the metallurgy of Pb, Hg, Cu, etc.
These methods are discussed below :
(i) Reduction with carbon and carbon monoxide
The oxides of metals like zinc, copper, tin, lead, etc. can be reduced by using carbon as reducing agent. The process of extraction of metal by reduction of its oxide with carbon (in the form of charcoal, coke or carbon monoxide) is called smelting.
The roasted ore is mixed with a suitable amount of carbon (coke or coal) or carbon monoxide and heated to a high temperature above the melting point of the metal in a furnace. Carbon or carbon monoxide (produced by incomplete combustion of carbon) reduces the oxide to free metal.
MxOy + yC —-> хМ + yCO
SnO2 + 2 C ——> Sn + 2 CO
ZnO + C —–> Zn + CO
PbO + C —–> Pb + CO
Fe2O3 + 3C —–> 2 Fe + 3CO
Fe2O3 + 3CO —–> 2 Fe + 3CO2
FeO + CO ——–> Fe + CO2
MnO2 + 2 C ——> Mn + 2 CO
Mn2O3 + 3 C ——> 2 Mn + 3 CO
ZnO + CO —-> Zn + CO2
The carbon reduction process is generally carried out in a blast furnace.
Different chemical reactions occur at different temperatures (zones) in the blast furnace.
(ii) Reduction by hydrogen
Certain metal oxides are reduced by hydrogen. Because of the inflammable nature of hydrogen, it is used in very few cases.
For example: molybdenum and tungsten oxides are reduced by a current of hydrogen at higher temperature (1270 – 1470 K).
WO3 +3 H2 ——> w + 3 H2O
MoO3 + 3 H2 ——> Mo + 3 H2O
NiO + H2 ——> Ni + H2O
(iii) Reduction by aluminium
Many oxides, like Cr2O3, Mn3O4, etc. are not reduced easily by carbon or CO. These metal oxides are reduced by strongly electropositive metals such as aluminium. The process of reduction of a metal oxide to the metal with the help of aluminium powder is called aluminothermy.
For example,
Cr2O3 + 2 Al ——> 2 Cr + Al2O3
3 Mn3O4 + 8 Al —–> 4 Al2O3 + 9 Mn
The above reaction is highly exothermic and therefore, the metals are produced in the molten state. The above process is also known as Goldschmidt thermite process.
Similarly, Fe2O3 can be reduced to metallic iron by aluminium :
Fe2O3 + 2 Al ——–> 2 Fe + Al2O3
The molten iron produced by thermite process can be used to weld broken parts of heavy machinery of all kinds such as rail, girders, etc. This process is also called thermite welding.
(iv) Reduction by sodium or magnesium or calcium or hydrogen
Certain metal halides are reduced to pure metal state by reduction with Na ,Mg or Ca, in a closed vessel on heating.
For example: titanium, zirconium, or vanadium metals are obtained by reduction of their halides with Na or Mg at higher temperatures (1070-1170K).
TiCl4 +4 Na —–> Ti + 4 NaCl
VCl4 + 2 Mg ——> V + 2 MgCl2
(v) Reduction with water gas
Nickel oxide (NiO) is reduced to nickel by heating carefully in a tower at 600 K in which a current of water gas (CO + H2) is passed. In this case both CO and H2, present in water gas act as agents.
NiO + CO —> Ni + CO2
NiO + H2 —> Ni + H2O
(vi) Self reduction or Auto-reduction
This is used for the reduction of sulphide ores of Pb, Hg and Cu. In this case, no reducing agent is required. The metal is obtained either by simple roasting or by the reduction of its partly oxidized form.
For example: Mercury is directly obtained by the roasting of its ore cinnabar (HgS) in air as:
HgS + O2 ——> Hg + SO2
HgS + 3 O2 —–> 2 HgO + 2 SO2
2HgO —-> 2 Hg + O2
2HgO + HgS —-> 3 Hg + SO2
Copper is also obtained by reducing Cu2S by partly converted Cu2O in this way during smelting.
Cu2S + 2Cu2O ——>6Cu + SO2
Lead is obtained from its sulphide ore (PbS) by heating in a supply of air at 770-970 K, when the ore gets converted into oxide and sulphate as :
2PbS + 3O2 —–> 2PbO + 2SO2
Galena
PbS + 2 O2 ——> PbSO4
Then the supply of air is stopped and a little lime added and the mixture is heated at a higher temperature (1070 K). The lead sulphide and lead sulphate get reduced to lead as
PbS + 2PbO ——> 3Pb + SO2
PbS + 2PbSO4 ——> 2Pb + 2SO2
In this case, lime acts as flux and removes SiO2, as slag, CaSiO3.
2HgS + 3 O2 ——> 2 HgO + 2 SO2
2HgO + HgS —> 3 Hg + SO2
Cu2S + 2 Cu2O ——-> 6Cu + SO2
All roasting processes are carried out in reverberatory furnace.
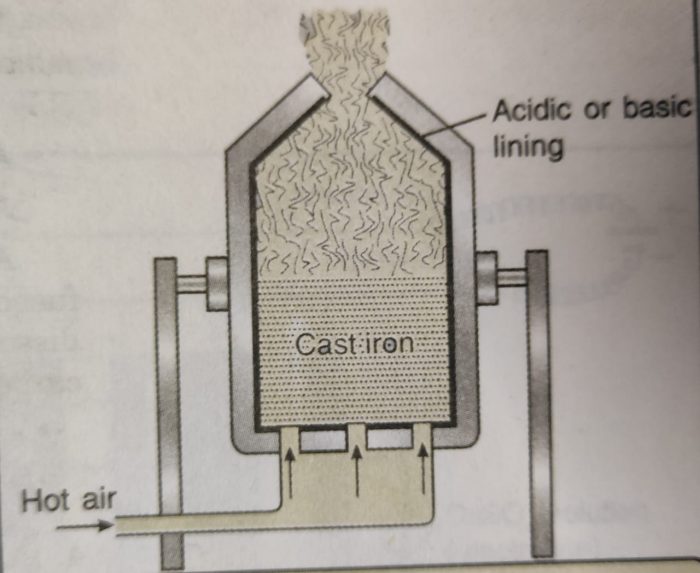
Two common types of converters are:
(i) Bessemer converter: It is a specially designed large pear-shaped furnace called Bessemer converter.
1) It is made of steel and lined with silica bricks.
2) The converter is mounted on horizontal pivots around which it can be tilted.
3) The impure metal is melted and a blast of hot air under pressure is passed through it.
4) The oxygen oxidises the impurities and raises the temperature to about 2173 K.
5) Pig iron and copper are purified by this method.
(ii) The Pierce-Smith converter.
1) It consists of a large horizontal steel drum resting upon rollers.
2) Rolls of steel tubes (called tuyeres) pass into the converter and are connected to an air duct.
3) Air is forced into the molten bath of crude metal.
4) The process provides its own heat due to the oxidation of impurities and the temperature rises to about 2673 K.
Some other Methods of Reduction
(1) Reduction by precipitation (Hydro-metallurgy) or Displacement method
Some metals are reduced by displacement by a more reactive metal from their complexes.
For example: Metals like gold, silver, etc. are extracted from their complex salt solutions by more electropositive zinc metal.
The ore is dissolved in some suitable solution to form their soluble complexes. The metal ion is then precipitated by adding zine dust. The process of extraction of metals by dissolving the ore in a suitable chemical reagent and the precipitation of the metal by more electropositive metal is called hydrometallurgy.
For example: Concentrated argentite, Ag2S is first treated with a dilute solution of NaCN to form the soluble complex, sodium dicyanoargentate (I).
Ag2S + 4NaCN —–> 2Na[Ag(CN)2] + Na2S
The solution is decanted off and made alkaline by adding NaOH and then treated with zinc or aluminium to precipitate silver.
2Na[Ag(CN)2] + Zn ——–> 2Ag + Na2[Zn(CN)4)]
Na2[Zn(CN)4] + 4NaOH ——->Na2ZnO2 + 4NaCN
Gold is also precipitated from its complex salt solution in a similar way.
2K [Au(CN)2] + Zn ——> K2[Zn(CN)4] + 2Au
Metals such as Ti, Zr, Ta etc. are also obtained by reducing their complex salts with alkali metals or Al.
K2TiF6 + 4K —–> 6KF + Ti
K2ZrF6 + 2Al ——> 2AlF3 + 2K + Zr
(2) Electrolytic Reduction
Certain highly electropositive metals such as alkali metals of groups 1 and 2 alkaline earth metals and aluminium, etc. are commonly extracted by the electrolysis of their fused salts.
The process of extraction of metals by electrolysis process is called electrometallurgy.
For example: sodium is extracted from fused sodium chloride by electrolysis as:
NaCl ——–> Na+ + Cl¯
At cathode
Na+ + e¯ —> Na
At anode
Cl¯ —–> Cl + e¯
Cl + Cl ——-> Cl2
The sodium metal is liberated at cathode and collected. Similarly, magnesium is prepared by the electrolysis of MgCl2
MgCl2 ⇔ Mg2+ + 2Cl¯
At cathode
Mg2+ + 2e‾ —-> Mg
At Anode
2 Cl‾ – 2e‾ —-> Cl2
Aluminium is obtained by the electrolysis of fused aluminium oxide. Since fused aluminium oxide (alumina) is not an electrolyte, it is made electrolyte by dissolving in fused cryolite (Na2AlF6) or Na3AlCl6 , and then electrolysed using carbon electrodes. Molten aluminium collects at cathode.
Al3+ + 3e¯ ———> Al
thank you so much , this is really work me a lot