Contents
Occurrence and Extraction of Copper
Occurrence of Copper
It occurs as native copper as well as in combined state.The main ores of copper are:
(i) Copper glance Cu2S
(ii) Copper pyrites CuFeS2
(iii) Malachite Cu(OH)2CuCO3
(iv) Cuprite or (Rubby copper) Cu2O
(v) Azurite 2CuCO3.Cu(OH)2
Extraction of Copper
It is mainly extracted from copper pyrites (CuFeS2). The sulphide ore is usually of very low grade and contains iron sulphide, gangue and smaller quantities of arsenic, antimony, selenium tellurium, silver, gold and platinum.
The various steps involved in the extraction are:
1) Crushing and concentration
The ore is crushed in jaw crushers and then finally powdered.
It is concentrated by froth floatation process.
In this process, the finely powdered ore is mixed with water and some pine oil in a tank.
The mixture is agitated by blowing compressed air into it.
The particles are preferentially wetted by oil and rise to the surface of the tank in the form of a froth (a foam) from where these are skimmed off.
The silicious and earthy impurities are preferentially wetted by water and sink to the bottom of the tank.
2) Roasting
The concentrated ore is roasted i.e., heated strongly in the presence of excess air in a reverberatory furnace. During roasting the following changes occur:
a) Moisture is removed from the ore and it becomes dry.
b) The impurities of sulphur, arsenic, antimony and phosphorus are removed as their volatile oxides.
S + O2 —> SO2
P4 + 5O2 —–> 2P2O5
4As + 3O2 → 2As2O3
4Sb + 3O2 → 2Sb2O3
(iii) Copper pyrites is converted to ferrous sulphide (FeS), cuprous sulphide(Cu2S) which are partially oxidised
2CuFeS2 + O2 —–> Cu2S + 2FeS + SO2
Copper pyrites
2FeS + 3O2 → 2FeO + 2SO2
2CuS + 3O2 → 2Cu2O + 2SO2
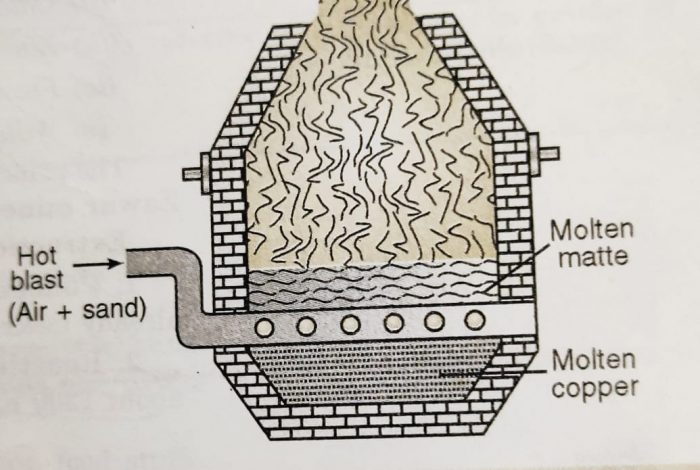
3) Smelting
The roasted ore is mixed with some powdered coke and sand and is heated strongly in a blast furnace.
The blast furnace is made up of steel and is lined inside with fire bricks. A blast of hot air is introduced at the lower part of the furnace.
The following changes occur during smelting :
(a) Most of the ferrous sulphide gets oxidised to ferrous oxide which combines with silica (flux) to form fusible slag.
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → FeSiO3
Ferrous silicate (slag)
The slag being lighter floats and forms the upper layer. It is removed through the slag hole from time to time.
(b) During roasting or in the blast furnace if any oxide of copper is formed, it combines with FeS and is changed back into its sulphide.
2Cu2S + 3O2 → 2Cu2O + 2SO2
Cu2O + Fes → Cu2S + FeO
Ferrous oxide thus formed again combines with silica to form more slag.
FeO+ SiO2 —–> FeSiO3
As a result of smelting, two separate layers are formed at the bottom of the furnace. The upper layer consists of slag and is removed as a waste. The lower layer of molten mass contains mostly cuprous sulphide and some traces of ferrous sulphide. It is called matte and is taken out from the taping hole at the bottom.
4) Bessemerisation
The molten matte from the blast furnace is transferred into a Bessemer converter.
The vessel is made of steel and is lined inside with lime or magnesium oxide. A hot blast of air mixed with sand is blown into the molten matte. During this process :
(i) traces of ferrous sulphide present in the matte is oxidised to FeO which combines with silica to form slag.
2FeS + 3O2 —-> 2FeO + 2SO2
FeO + SiO2 —-> FeSiO3
(ii) copper sulphide is partially oxidised to cuprous oxide which further reacts with remaining copper sulphide to form copper and sulphur dioxide.
2 Cu2S + 3O2 → 2Cu2O + 2SO2
Cu2S + 2Cu2O —-> 6 Cu + SO2
After the reaction has been completed, the converter is tilted and the molten copper is poured into sand moulds. On cooling, sulphur dioxide, nitrogen and oxygen escape from the metal. The copper thus, obtained is about 99% pure and is known as blister copper. The name blister comes from the fact that as the metal solidifies, the dissolved SO, escapes producing blisters on the metal surface.
5) Refining
a) Poling: The blister copper is purified by heating it strongly in a reverberatory furnace in the presence of excess of air. The impurities are either removed as volatile oxides or converted into slag.
Some of the copper also changes to cuprous oxide. This is reduced back to copper by stirring the molten metal with green poles of wood. The hydrocarbons present in these freshly cut poles reduce cuprous oxide to copper which is about 99.5% pure.
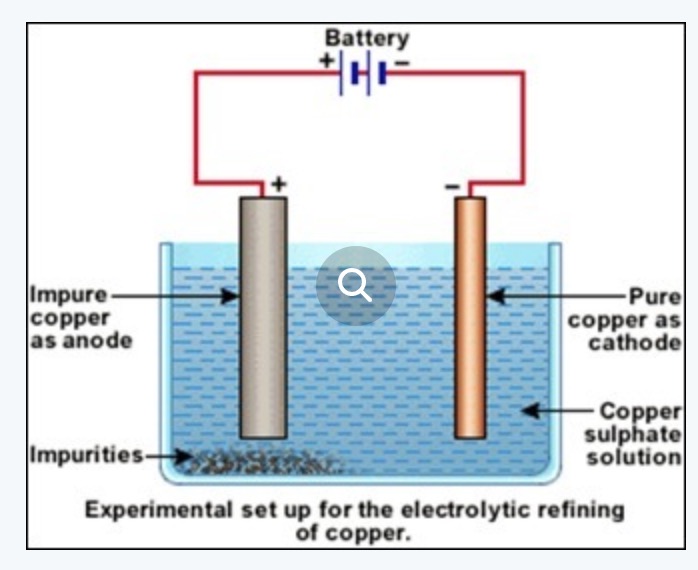
b) Electrolytic refining: In this method, a thin sheet of metal is made as cathode and the block of crude metal is made as anode.
Both the electrodes are placed in an acidified copper sulphate solution. When electric current is passed through the solution impure copper from anode goes into the solution and pure copper from the solution gets deposited on the cathode.
At anode Cu -2e¯ ——-> Cu2+
At cathode Cu2+ + 2e‾ →Cu
The impurities of zinc, nickel, iron, etc. get collected below the anode as anode mud.
Prolonged exposure of copper pyrites to air and rain leads to the formation of dilute solution of copper sulphate. Copper can be precipitated from this solution by the addition of scrap iron. It is then refined electrolytically.
Occurrence and Extraction of Zinc
Zinc does not occur in native form because it is a reactive metal. The main ores of zinc are:
(i) Zinc blende ZnS
(ii) Calamine ZnCO3
(iii) Zincite ZnO
(iv) Franklinite ZnO.Fe2O3
(v) Willemite Zn2SiO4
The principal ore of zinc is zinc blende.
Extraction: Zinc can be extracted from zinc blende by the following steps:
1) Concentration: The ore is concentrated by froth floatation process.
2) Roasting: Concentrated ore is roasted in the presence of excess of air at about 1200 K to convert zinc sulphide into zinc oxide.
2ZnS + 3O2 ——> 2ZnO + 2SO2
Sulphur dioxide gas evolved in this process may be used in the manufacture of sulphuric acid.
3) Reduction: Zinc oxide is reduced to zinc by heating with crushed coke at 673 K in vertical fire clay retorts.
ZnO + C —–> Zn + CO
The vapours of zinc formed are collected and condensed.
4) Refining: The impure metal is refined by fractional distillation or by electrolytic method.
(i) By fractional distillation: Impure zinc contains impurities of cadmium (b.p. = 1073 K), lead (b.p. 2024 K) and iron (b.p. 3273 K). The boiling point of zinc is 1183 K. The impure metal is distilled when zinc and cadmium with low boiling points distil over leaving behind lead and iron.
The boiling points of zinc and cadmium are also different and therefore, the mixture of zinc and cadmium is again subjected to fractional distillation when low boiling cadmium distils leaving behind zinc metal in the distillation flask.
(ii) By electrolytic refining: In this process, the impure zine is made anode while a plate of pure zinc is made the cathode. The electrolyte is zinc sulphate containing a small amount of dilute sulphuric acid. On passing current, zinc from the electrolyte is deposited at the cathode while an equivalent amount of zinc from anode goes into the electrolyte. Therefore, pure zinc is obtained on cathode.
Notes are very simple, easy to understand and contain complete content.Excellent work.
Very enjoyable notes thank you for helping me!! wish you a good long life