Contents
Electrode Potential
The flow of electric current in an electrochemical cell indicates that a potential difference exists between two electrodes.
When an electrode say copper, is immersed in a solution of its ions, then either of the following three possibilities can take place:
a) The metal ions (Cu2+) may collide with the electrode and do not undergo any change.
b) Cu2+ ions may collide with the electrode, gain electrons and get converted into metal atoms (i.e. the ions are reduced)
Cu2+ + 2e¯ ——> Cu (Reduction)
c) Cu atoms on the electrode may lose electrons to the electrode and become Cu2+ ions and go into the solution (i.e., oxidation occurs)
Cu ——> Cu2+ + 2e¯ (Oxidation)
If the metal has relatively high tendency to get oxidised, its atoms will lose electrons readily and form Cu2+ ions, which go into the solution. The electrons lost on the electrode would be accumulated on the metal electrode and the electrode acquires a slight negative charge with respect to the solution.Some of the Cu2+ ions from the solution will take up electrons and become Cu atoms. After some time, an equilibrium will be established as
Cu(s) ⇔ Cu2+ + 2e¯
When such an equilibrium is attained, it results in separation of charges.
If the metal ions have relatively greater tendency to get reduced, they will take electrons from the electrode. As a result, a net positive charge will be developed on the electrode with respect to the solution. This will also result into separation of charges.
Due to separation of charges between the electrode and the solution, an electrical potential is set up between metal electrode and its solution.
The electrical potential difference set up between the metal and its solution is known as electrode potential. The electrode potential is a measure of tendency of an electrode in a half cell to gain or lose electrons. The electrode potential may be of two types:
1) Oxidation Potential
The tendency of an electrode to lose electrons or to get oxidised is called its oxidation potential. Thus, oxidation potentials give the tendency of an electrode to lose electrons, i.e.
M(s) ⇔ Mn+ (aq) + ne¯
Zn⇔ Zn2+ (aq) + 2e¯
H2 ⇔ 2H+ (aq) + 2e¯
2) Reduction Potential
The tendency of an electrode to gain electrons or to get reduced is called its redution potential. Thus, reduction potentials give the tendency of an electrode to gain electrons, i.e.
Mn+ (aq) + ne¯ ⇔ M(s)
Cu2+ ( aq) +2e¯ ⇔ Cu(s)
2H+ (aq) + 2e¯ ⇔ H2
The oxidation potential is the reverse of reduction potential.
For example: if reduction potential of Zn is- 0.76 volts, its oxidation potential is +0.76 volts
The half cell reactions are always written as reduction half reactions and their potentials are represented as reduction potentials. These are called electrode potentials.
The electrode potential depends upon
1) the nature of the metal and its ions,
2) concentration of the ions in the solution, and
3) temperature
E.M.F. or Cell Potential of a Cell
Electrochemical cell consists of two half cells. The electrodes in these half cells have different reduction potentials. Therefore, they have different tendency to lose or gain electrons.
The electrode having higher reduction potential will have higher tendency to gain electrons whereas the electrode having lower reduction potential will have lesser tendency to gain electrons, rather it loses electrons. As a result of this potential difference there is a flow of electrons from the electrode with a lower reduction potential (higher tendency to lose electrons) to the electrode with higher reduction potential.
The difference between the electrode potentials of the two electrodes constituting an electrochemical cell is known as electromotive force or cell potential of a cell. The potential difference is expressed in volts.
The reduction occurs at cathode and oxidation occurs at anode.
e.m.f. = Reduction potential of cathode – Reduction potential of
or Ecell= E(cathode) – E(anode)
Cathode is written on right hand side and anode on the left hand side. Therefore, e.m.f. of a cell may also be written as :
Ecell = E (Right) – E (Left)
Thus, e.m.f. of a cell may be defined as the potential difference between two electrodes of the cell when either no or negligible current is allowed to flow in the circuit.
The e.m.f. of the cell is measured with the help of a potentiometer. It depends upon the nature of the electrodes, temperature and the concentrations of the solutions in the two half cells.
Standard Electrode Potential
Since a half cell in an electrochemical cell can work only in combination with the other half cell and does not work independently, it is not possible to determine the absolute electrode potential of an electrode. We can find only the relative electrode potential.
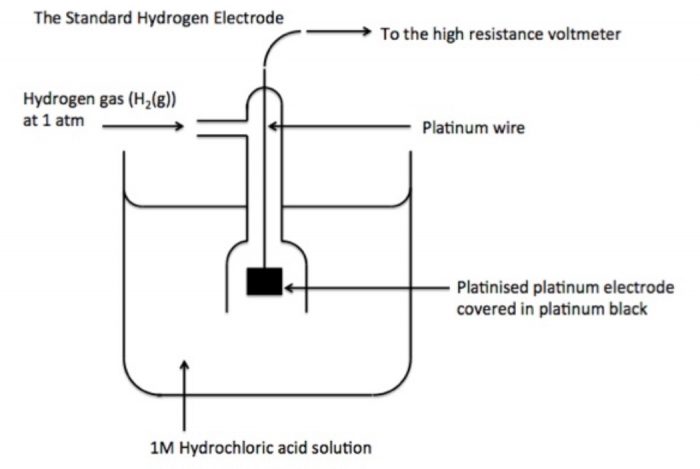
This difficulty can be solved by selecting one of the electrodes as a reference electrode and arbitrarily fixing the potential of this electrode as zero. For this reversible hydrogen electrode has been universally accepted as a reference electrode. It is called standard hydrogen electrode (S.H.E.) or normal hydrogen electrode (N.H.E.).
Standard hydrogen electrode : It consists of platinum wire sealed in a glass tube and has a platinum foil attached to it. The foil is coated with finely divided platinum and acts as platinum electrode. It is dipped into an acid solution containing H ions in 1 M concentration (1 M HC). Pure hydrogen gas at 1 bar (atmosphere) pressure is constantly bubbled into solution at constant temperature of 298 K. The surface of the foil acts as a site for the reaction.
If S.H.E. acts as anode
H2(g) ——-> 2H+ + 2e¯
If S.H.E. acts as cathode
2H+ + 2e¯ ——–>H2(g)
This standard hydrogen electrode is also regarded as reversible electrode
H2(g) ⇔ 2H+ + 2e¯
The electrode potential of an electrode can be determined by connecting this with a standard hydrogen electrode. The electrode potential of the standard hydrogen electrode is taken as zero. The electrode potential of a metal electrode as determined with respect to a standard or normal hydrogen electrode is called standard electrode potential (E°)
Measurement of the Standard Electrode Potential
To measure the standard electrode potential of a metal electrode, 1.0 M solution of the electrolyte is taken in a beaker and a metal electrode is dipped in it. This constitutes the metal-metal ion electrode, M/Mn+ (aq). This half cell is connected to S.H.E. through a salt bridge. The electrodes are connected to a voltmeter. From the measured e.m.f. of the cell, the standard electrode potential of the half cell is calculated.
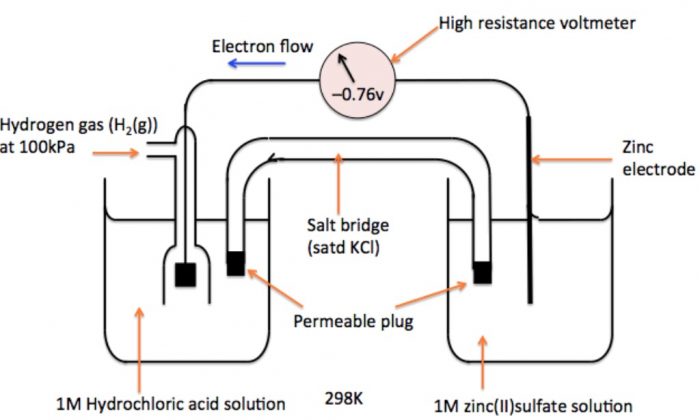
a) Measurement of Electrode Potential of Zn2+| Zn Electrode
An electrode consisting of zinc rod immersed in 1M solution of ZnSO4, is combined with S.H.E. In this case, the electrons flow from zinc electrode to hydrogen electrode and therefore, the zinc electrode acts as anode and S.H.E. acts as a cathode. The cell may be represented as:
Zn | Zn2+ (1 M) || H+ (1 M) | H2 (1 atm), Pt
The cell potential has been measured to be 0.76 V. Now e.m.f. of cell,
Eø = Eø R – Eø L
Eø = Eø (H+|H2) – Eø (Zn2+| Zn)
0.76 =0 – Eø (Zn2+| Zn)
Eø (Zn2+| Zn) = -0.76 V
Thus, by combining with S.H.E. reduction potential value of Zn has been found to be 0.76 V.
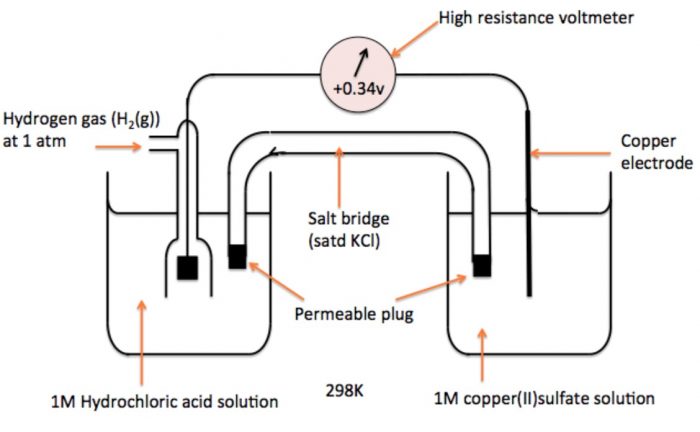
b) Measurement of Electrode Potential of Cu2+| Cu Electrode
To measure electrode potential of copper-copper ions, a cell consisting of copper electrode immersed in 1 M CuSO4 solution and S.H.E.
The hydrogen has greater tendency to lose electrons in comparison to copper. Therefore, oxidation occurs at hydrogen electrode and reduction occurs at copper electrode. Consequently, the hydrogen electrode acts as anode and copper electrode acts as cathode.
Thus, the cell may be represented as
Pt, H2 (1 atm) | H+ (1M) || Cu (1M) | Cu
The cell potential has been measured to be 0.34 V.
Eøcell = Eø R – Eø L
Eøcell = Eø (Cu2+|Cu) – Eø (H+ | H2)
0.34 = Eø (Cu2+|Cu) – 0
Eø (Cu2+|Cu) = 0.34 V
Thus, the standard electrode potential of copper is + 0.34 V
The electrode at which reduction occurs with respect to S.H.E. has positive reduction potential while the electrode at which oxidation occurs with respect to S.H.E. has negative reduction potential.
That was really helpful and informative. Thank you for putting in lots of efforts!