Contents
Isomerism
Isomers can be broadly classified into two major categories :
Structural isomers
Stereoisomers
(A) Structural isomerism
(1) Ionisation isomerism
For example: There are two isomers of the compound of the formula Co(NH3)5BrSO4
The structures of the two compounds and their mode of ionisation are :
[CoBr(NH3)5]SO4 → [CoBr(NH3)5]2+ + SO42-
cobalt(III) sulphate Gives test of SO42- ions
[CoSO4(NH3)5]Br → [CoSO4(NH3)5]+ + Br¯
Other compounds showing this type of isomerism are:
(i) [CoCl2(NH3)4]NO2 and [CoCl(NO2)(NH3)4]Cl
(ii) [Co(NO3)(NH3)]SO4 and [Co(SO4)(NH3)5]NO3
(iii) [PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2
(iv) [CoCl(NO2)(NH3)4]Cl and [CoCl2(NH3)4]NO2
(2) Solvate or Hydrate isomerism
It is also known as hydrate isomerism where water is involved as a solvent.Thus, hydrate isomers differ in the number of water molecules present as ligands or as molecules of hydration.
In type of isomerism water molecules may occur inside and outside the coordination sphere as a coordinated group or a water of hydration.
These are :
(i) [Cr(H2O)6]Cl3 : It does not lose water when treated with conc. H2SO4 and three chloride ions are precipitated with AgNO3.
(ii) [CrCl(H2O)5]Cl2⋅H2O
(iii) (CrCl2(H2O)4]Cl.2H2O : It loses two water molecules on treatment with conc. H2SO4 dark green and one Cl¯ ion is precipitated with AgNO3.
Similarly, the following two isomers are hydrate isomers :
(3) Coordination isomerism
The examples are:
(ii) [Cu(NH3)4[PtCl4] and [Pt(NH3)4][CuCl4]
This type of isomerism is also shown by compounds in which the metal ion is the same in both cationic and anionic complexes.
For example :
(4) Linkage isomerism
For example: In NO2¯ ion, the nitrogen atom as well as the oxygen atom can donate their lone pairs. This gives rise to isomerism.
If oxygen donates its lone pair, a different compound (although having the same molecular formula) is obtained. If the bonding is through N, the ligand is named as nitrito-N(or nitro) and if it is through O, it is named as nitrito-O (or nitrito).
For example: Jorgensen discovered such behaviour in the complex [(Co(NH3)5(NO2)]Cl. He prepared two different pentaamminecobalt(II) chloride each containing the NO2 group in the complex ion. These are:
The unidentate ligands which can bind to the central atom through two donor atoms are also called ambidentate ligands.
Other examples of ligands are:
(B) Stereoisomers
(1) Geometrical isomerism
(a) Geometrical isomerism in complexes of coordination number 4
(1) Square planar complexes of the type MA2X2 , MA2XY, MABX2, MABXY can exist as geometrical isomers (Here A and B are neutral ligands such as H2O, NH3, CO, NO, C5H5N whereas X and Y are anionic ligands such as Cl¯, NO2‾, CN¯, SCN¯ etc.)
Example:
(i) [PtCl2(NH3)] exists in cis and trans formas:
![[Pt(Cl2(NH3)2]](https://classnotes.org.in/wp-content/uploads/PtCl2NH32-700x245.jpeg)
(ii) [PtCl(C5H5N)2 (NH3)] exists in cis and trans form as:
![[PtCl(C5H5N)2(NH3)]](https://classnotes.org.in/wp-content/uploads/PtClC5H5N2NH3-700x198.jpeg)
(iii) Square planar complexes of the type MABCD show three isomers. The structures of these isomers can be written by fixing the position of one ligand (say A) and placing the other ligands B, C and D trans to it.
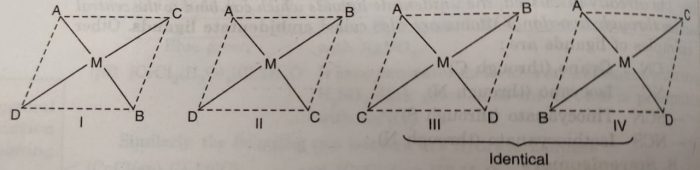
The complex [Pt(NO2)(py) (NH2OH)(NH3)]+ exists in three geometrical isomers as represented below:
![[Pt(NO2)py (NH2OH)(NH3)]+](https://classnotes.org.in/wp-content/uploads/PtNO2py-NH2OHNH3-700x141.jpeg)
(iv) Geometrical isomerism cannot occur in complexes of the type MA4 , MA3B or MAB3 because all possible spatial arrangements for any of these complexes will be exactly equivalent.
(v) The square planar complexes containing unsymmetrical bidentate ligands such as [M(AB)2] also show geometrical isomerism. For example, the complex [Pt(gly)2] where gly = NH2CH2COO¯ exists in cis and trans form :
![cis and trans isomers of [Pt)gly)2]](https://classnotes.org.in/wp-content/uploads/cis-and-trans-isomers-of-Ptgly2-700x186.jpeg)
(vi) Geometrical isomerism is also shown by bridged binuclear complexes of the type M2A2X4. For example: the complex [PtCl2 P(C2H5)3]2 exhibits geometrical isomers as :
![cis [PtCl2 P(C2H5)3]2](https://classnotes.org.in/wp-content/uploads/cis-PtCl2-PC2H532-700x213.jpeg)
![Trans [PtCl2 P(C2H5)3]2](https://classnotes.org.in/wp-content/uploads/Trans-PtCl2-PC2H532-700x253.jpeg)
(b) Geometrical isomerism in complexes of coordination number 6
(1) The octahedral complexes of the type MA4X2, MA2X4 , MA4XY, etc. exhibit geometrical isomerism. Some common examples are :
An octahedral complex [CoCl2(NH3)4]+can exist as cis- and trans- isomers :
![cis and trans isomers of [CoCl2(NH3)4]+](https://classnotes.org.in/wp-content/uploads/cis-and-trans-isomers-of-CoCl2NH34.jpg)
Similarly, the complex [Fe(CN)4(NH3)2]¯ can exist as cis- and trans isomers.
![cis and trans isomer of [Fe(CN)4(NH3)2]-](https://classnotes.org.in/wp-content/uploads/cis-and-trans-isomer-of-FeCN4NH32--700x335.jpeg)
(2) Octahedral complexes of the type [MA3B3] like [Co(NO2)3(NH3)3] also exist in two geometrical isomers.
![fac and mer isomers of [Co(NO2)3(NH3)3]](https://classnotes.org.in/wp-content/uploads/fac-and-mer-isomers-of-CoNO23NH33-300x201.jpg)
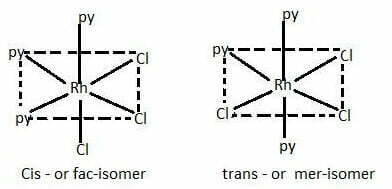
When the three ligands (with same donor atoms) are on the same triangular face of the octahedron, the isomer is called facial or fac isomer.
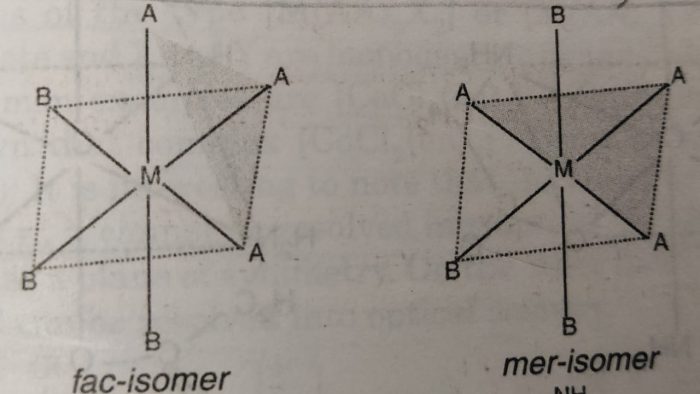
When the three ligands are on the same equatorial plane of the octahedron i.e., around the meridian of the octahedron, the isomer is called meridional or mer isomer.
In facial isomer, the three ligands are at the corners of a triangular face while in meridional isomer, the three ligands are at the three corners of a square plane.
[RhCl3(py)3] exist as fac and mer isomers.
(3) Octahedral complexes having didentate ligands of the type M(AA)2X2and M(AA)2XY can also exist as cis and trans isomers, where AA represents a symmetrical bidentate ligand such as ethylenediamine (en), oxalate ion (ox).For example, an octahedral complex [Co(en)2Cl2]+ exists as two isomers :
![[Co(en)2Cl2]+](https://classnotes.org.in/wp-content/uploads/Coen2Cl2-700x289.jpeg)
(4) Octahedral complexes having six different ligands of the type M(ABCDEF) would exhibit geometrical isomerism. These isomers may be written by fixing a ligand at one position and then placing the other ligands trans to it.
15 different isomers are possible for such type of complexes. The only compound of this type that has been prepared is [Pt(Br)(Cl)()(NO2)(py)(NH3)].
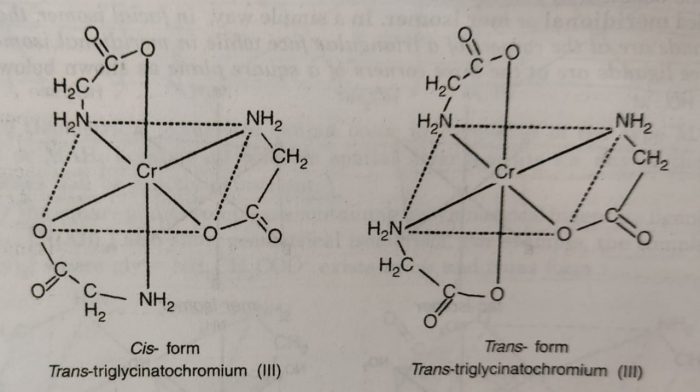
(5) The complexes containing unsymmetrical bidentate ligands also show geometrical isomerism. For example: the complex triglycinatochromium (III), [Cr(gly)3], where gly is H2NCH2COO¯, exists in cis and trans forms.
(2) Optical isomerism
The isomer which rotates the plane of polarised light to the right is called dextro rotatory designated as (d) and the one which rotates the plane of polarised light to the left is called laevo rotatory designated as (l). A 1:1 equilibrium mixture of d and l isomers gives a net zero rotation and is also called racemic mixture.
The d and l isomers are mirror images of each other just as left hand is mirror image of the right hand. These mirror images compounds are non-superimposable on each other and do not possess the plane of symmetry.
The common examples of complexes showing optical isomerism are octahedral complexes having bidentate ligands.
(i) Complexes of the type M(AA)3 (where AA is symmetrical bidentate ligands) such as [Co(en)3]3+ and [Cr(ox)3]3- exist as optical isomers.
![[Co(en)3]3+ and [Cr(ox)3]3-](https://classnotes.org.in/wp-content/uploads/Coen33-and-Crox33--700x445.jpeg)
Some common examples are [CoCl2(en)2]+, [RhCl2(en)2]+, etc.
![cis-[CoCl2(en)2]+](https://classnotes.org.in/wp-content/uploads/cis-CoCl2en2-1-700x302.jpeg)
The trans form does not show optical isomerism, i.e., it cannot be resolved into optical isomers. The reason is that the molecule has a plane of symmetry. On the other hand, the cis-isomer is unsymmetrical and can be resolved into optical isomers.
![[PtCl2(en)2]2+](https://classnotes.org.in/wp-content/uploads/PtCl2en22-700x478.jpeg)
(iii) Complexes of the type [M(AA)X2Y2] containing one symmetrical bidentate ligand show optical isomerism. For example [CoCl2(en)(NH3)2]+ exists in d- and l- forms.
(iv) Octahedral complexes containing hexadentate ligands such as ethylenediaminetetracetato (EDTA) also show optical isomerism.
![Optical isomers of [Co(edta]-](https://classnotes.org.in/wp-content/uploads/Optical-isomers-of-Coedta--700x446.jpeg)
![trans[CoCl2(en)2]+](https://classnotes.org.in/wp-content/uploads/transCoCl2en2-700x281.jpeg)
excellent
Good amazing woooooow ❤️❤️❤️
It was really helpful