Expression for Equilibrium constant ( K )
The active mass of a pure solid is constant irrespective of its amount and if a pure liquid is present in excess, its active mass is also constant.In both the cases we put active mass equal to 1.
Molar concentration of a substance means mol L-1 of the substance which is obtained by dividing the amount of the substance in moles by the volume of the substance in litres.
Molar Conc. = Moles of the substance / Volume of the substance
Molar Conc. =( Mass of the substance / Molecular mass ) / Volume of the substance
Molar Conc. = Density of the substance/ Molecular mass of the substance
As density of a particular pure substance at a particular temperature is constant and molecular mass of the substance is also constant, therefore, molar concentration is constant.
For Homogeneous Equilibrium
CH3COOH ( l ) + C2H5OH ( l ) CH3COOC2H5 ( l ) + H2O ( l )
NH3 ( g ) + 5O2 ( g ) 4NO ( g ) + 6H2O ( g )
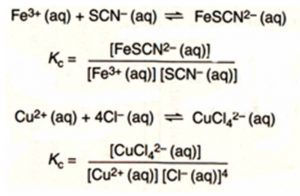
For Heterogeneous Equilibrium
Units Of Equilibrium Constant
Keq = ( mol L-1 ) c+d / ( mol L-1 ) a+b
Keq =( mol L-1 ) (c+d ) – (a + b )
Keq = (mol L-1 ) Δn
Kp = (atm) (c+d) / (atm)a+b
Kp = (bar) (c+d) / (bar)a+b
Kp = ( atm or bar )(c+d) – (a+b)
Kp = (atm)Δn or (bar)Δn
If Δn =0 i.e. number of moles of products = number of moles of reactants , Keq or Kp will have no units.
For Ex:
1) H2 ( g ) + I2 ( g ) 2HI ( g )
Δn =0 , Kc or Kp will have no units.
2) N2 ( g ) + 3H2 ( g ) 2NH3 ( g )
Δn = 2 – ( 1+ 3 ) = -2
The units will be ( mol L-1 )-2 or atm-2 or bar-2
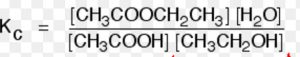
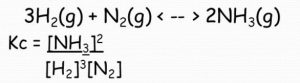
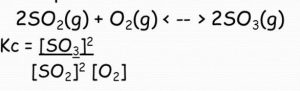
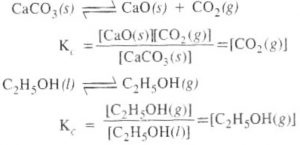
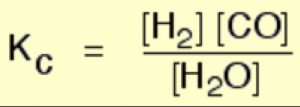
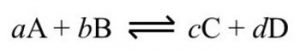
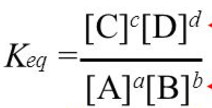
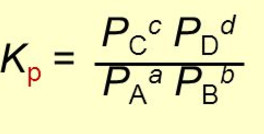
Leave a Reply