Characteristics Of Equilibrium Constant
(1) The value of the equilibrium constant for a particular reaction is always constant depending only upon the temperature of the reaction and is independent of the concentration of the reactant with which we start or the directions from which the equilibrium is approached.
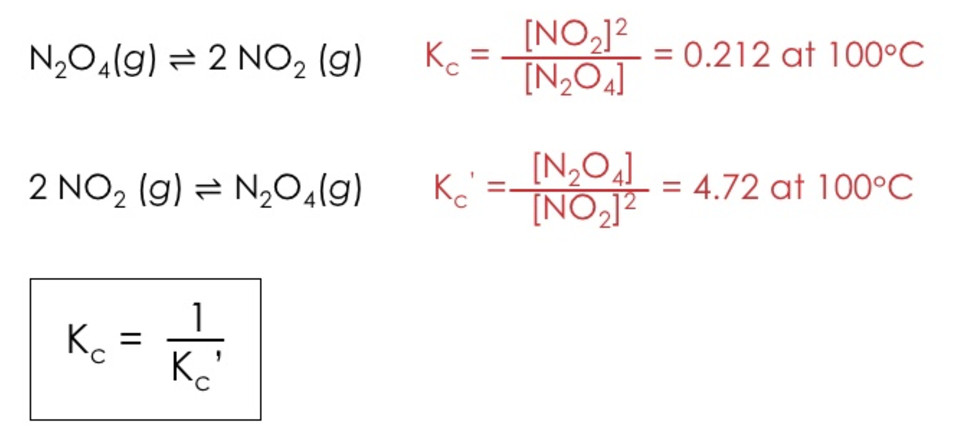
(2) If the reaction is reversed, the value of equilibrium constant is reversed.
(3) If the equation having equilibrium constant K is divided by 2 ,the equilibrium constant for the new equation is the square root of K.
(4) If the equation having equilibrium constant is multiplied by 2 ,the equilibrium constant for the new equation is a square of equilibrium constant.
(5) If the equation having equilibrium constant is written in 2 steps then equilibrium then K= K1 × K2
(6) The value of equilibrium constant is not affected by the addition of a catalyst
This is because the catalyst increases the speed of forward reaction and backward reaction to same extent.
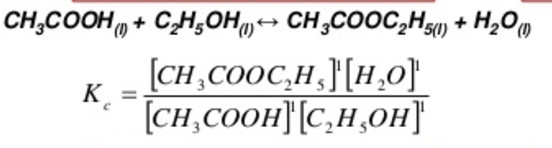
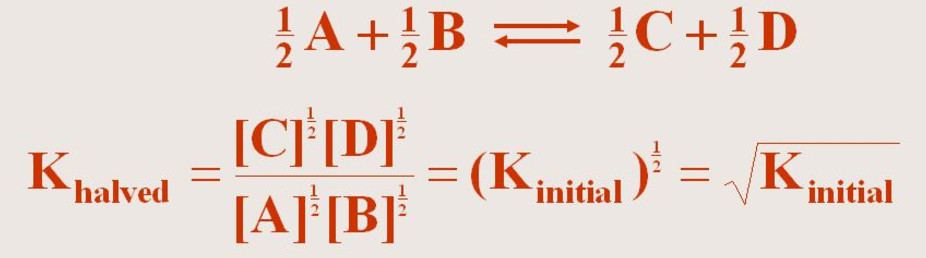
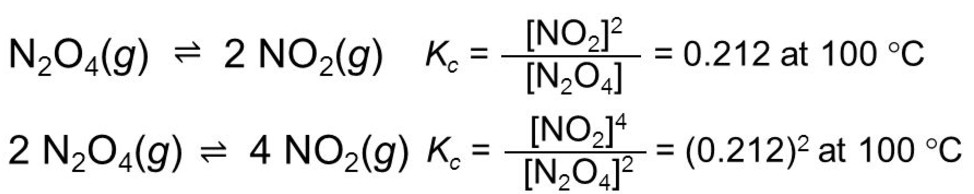
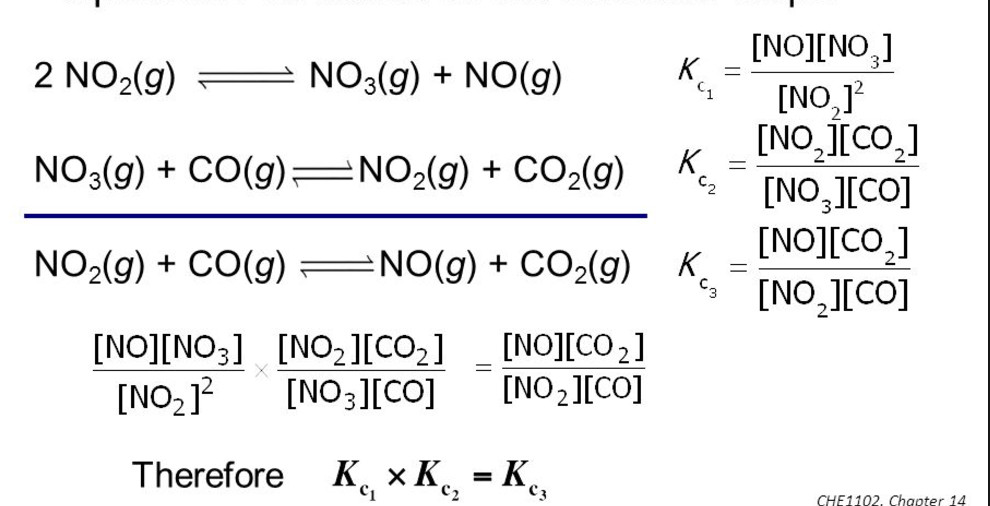
thanks for help in our study
Effact of concentration on equlibrium
You have no idea what your help meant to me .
I just want to say THANK YOU !
The explanation is good to understand
thank you mam for such good notes
Thank you so much madam for the help that you are doing. This is really boosting for those who has doubts in chemistry. God bless You.