Application of Equilibrium constant
1) Predicting the extent of reaction
The magnitude of the equilibrium constant gives an idea of the relative amount of the reactants and the products.
a) larger value of the equilibrium constant ( > 103 ) shows that forward reaction is favoured i.e. concentration of products is much larger than that of the reactants at equilibrium.
For Ex:
1) H2 ( g ) + Br2 ( g ) 2HBr ( g) Kc = 5.4 × 1018
2) H2 ( g ) + Cl2 (g ) 2HCl ( g ) Kc = 4 × 1031
3) H2 ( g ) + ½ O2 ( g ) H2O ( g ) Kc = 2.4 × 1047
This shows that at equilibrium , concentration of the products is very high , i.e. reaction go almost to completion.
b) Intermediate value of K ( 10-3 to 103 ) show that the concentration of the reactants and products are comparable.
For Ex:
1) Fe3+ (aq ) + SCN–( aq ) (Fe(SCN))2+ (aq) Kc = 138 at 298 K
2) H2 ( g) + I2 ( g ) 2HI ( g ) Kc = 57 at 700 K
c) low value of K ( < 10 -3) shows that backward reaction is favoured i.e. concentration of reactants is much larger than that of products i.e. the reaction proceeds to a very small extent in the forward direction.
For Ex:
1) N2 ( g ) + O2 ( g ) 2NO ( g ) Kc =4.8 × 10 -31 at 298 K
2) H2O ( g ) H2 ( g ) + ½ O2 ( g ) Kc = 4.1 × 10 -48
2) Predicting the direction of a reaction
At any stage of the reaction, other than the stage of chemical equilibrium, concentration ratio, as given by the expression for the law of chemical equilibrium , is called concentration quotient or reaction quotient.
It is usually represented by Qc or Q. Thus,
1) If Q=Kc , the reaction is in equilibrium
2) If Q > Kc , Q will tend to decrease so as to become equal to K. As a result, the reaction will proceed in the backward direction.
3) If Q < Kc, Q will tend to increase so as to become equal to K. As a result, the reaction will proceed in the forward direction.
3) Calculating equilibrium concentration
Knowing the initial concentration of reactants, equilibrium concentration of all the reactants and products can be calculated as:
1) Write the balanced equation for the reaction.
2) Assume x as the amount of the reactants reacted or a product formed.
3) Calculate the equilibrium concentration of each reactant and product from its stoichiometry of the equation.
4) Write expression for Kc or Kp.
5)Substitute equilibrium concentration and calculate x.
6) Check the result by substituting calculated values of equilibrium concentration to get the value of Kc or Kp
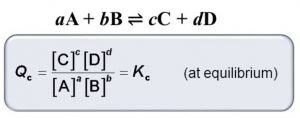
Good notes
Thank you mam
very very easy to study from this site thank you so much mam