Question 1 Name the particle used by Rutherford in his experiment to determine the structure of an atom?
Question 2 Explain the Rutherford’s scattering experiment?
Question 3 What observation were made by Rutherford’s in his experiment?
Question 4 Give the postulates of Rutherford’s model of an atom?
Question 5 State one drawback of Rutherford’s model of an atom?
Alpha Particles
Alpha particles is a positively charged particle having 2 units of positive charge and 4 units of mass. They are emitted from radioactive elements like Radium and Polonium. The fast moving alpha particles have considerable amount of energy. They can penetrate through the matter.
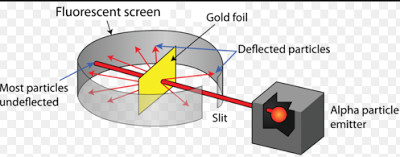
Experimental set Up
(1) He selected a thin gold foil.
(2) The fast moving alpha particles are allowed to strike a very thin gold foil in vacuum.
Observation
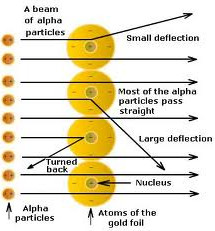
(1) Most of the alpha particles pass straight through the gold foil without any deflection from their original path.
(2) A few alpha particles are deflected through small angles and few are deflected through large angles.
(3) A very few alpha particles completely rebound on hitting the gold foil and turn back on their path.
Conclusion
(1) As most of the alpha particles pass straight through the gold foil without any deflection, it shows that there is lot of empty space in an atom.
(2) Some of the alpha particles are deflected through small and large angles shows that there is positive centre in the atom which repel the positively charged alpha particles.
(3) Very few alpha particles completely rebound on hitting shows that all positive charge and mass of the atom were concentrated in very small volume with the atom.
Rutherford Model of an atom
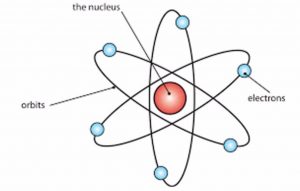
(1) An atom consist of positively charged, dense and very small nucleus containing protons and neutrons.The entire mass of an atom is concentrated in the nucleus.
(2) The nucleus is surrounded by negatively charged electrons. The electrons are revolving around the nucleus in circular paths at very high speed.These circular paths of the electrons are called orbits.
(3) An atom is electrically neutral because the number of protons and electrons is equal.
(4) The size of nucleus is very small as compared to size of atom.
Drawback of Rutherford Model of an Atom
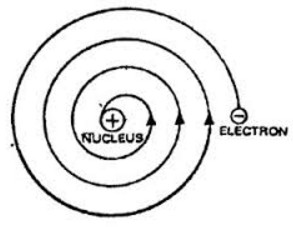
He does not explain the stability of an atom.In the Rutherford’s model of an atom, the negatively charged electron revolves around the positively charged nucleus in circular path. If an object moves in a circular path, the its motion is said to be accelerated. This means that motion of an electron revolving around the nucleus is accelerated. If a charged particle undergoes accelerated motion, then it must radiate energy continuously. Thus the energy of revolving electron will decrease gradually and their speed will also go on decreasing and ultimately the electrons should fall into the nucleus. This makes atom very unstable and hence the atom should collapse.
Thanks for the information you always give GOD BLESS
I like it
Thank you
thank you so much for the explaination. It was so difficult for me to understand the rutherford gold foil experiment. but u made it so easy to understand this concept.thank u sooo much.
Thanks for this explanation
Thank u so much
The picture of the atomic model you show at “Conclusion” after point 4) is actually Bohr’s model of the atom (since it has electrons on discrete energy levels). As you said, the electron orbiting around the nucleus would constantly lose energy so it would collapse into the nucleus eventually (Rutherford 1911). It was 1913 when Rutherford together with Bohr figured out that the electron can have stable orbits around the nucleus ONLY at certain “distances”, and nowhere else. However, an electron can jump between these stable orbits (also known as discrete energy levels – as you show in that picture). When the electron jumps “down” (i.e. closer to the nucleus) the process is called “de-excitation”, and it is usually accompanied by the emission of a photon (light). This model could finally explain the “Balmer series”, a.k.a. the emission spectrum of hydrogen.
We have made the changes as per your suggestions. Thank you so much for reading our website content so carefully and advising.
Keep Reading !!
Thanks for giving me a simple explanation
Thanks a lot for the information because of this I got very good mark in project making this what the best
Thanks a lot for the diagrams they helped me a lot
.
Thank for this kind of help
Thanks for simple explanation
It helps me more in my viva.
Nice brief about the rutherford model
Thanku