Question 1 Describe Thomson’s model of an atom?
Question 2 Which subatomic particle was not present in Thomson’s model of an atom?
Question 3 Why Thomson’s model is called as Plum pudding model of an atom?
Structure of an Atom
Dalton atomic theory suggested that atoms are indivisible(could not be broken into smaller particles)
But the discovery of subatomic particles inside an atom disproved this postulate of Dalton atomic theory.
After the discovery of these subatomic particles,it became necessary to find out how these particles are arranged inside the atoms.
J.J. Thomson was the first scientist to propose a model for structure of an atom.
Thomson Model of an atom
When J.J. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.
According to Thomson Model of an atom :
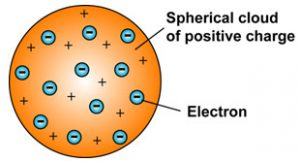
1) An atom consist of a sphere of positive charge with negatively charged electrons embedded in it.
2) The positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.IT has no overall positive and negative charge.
Thomson model of the atom is similar to that of a christmas pudding. The electrons embedded in a sphere of positive charge are like the dry fruits in a spherical christmas pudding. We can also compare Thomson’s model of an atom to a watermelon. The red edible part of watermelon represents the sphere of positive charge whereas the black seeds embedded in watermelon are like electrons.
Thank you so much
Can you help me to do thomson model of atom as an project
It is easy. Just draw A big circle
And just draw many electrons inside.andwhere you can show that positive eletrons are sphere and electrons are embedded in it.
Thank you for the help
ThANK U…..IT IS VERY EASY TO UNDERSTAND…
Thanks
It’s very easy
Thanks it is very easy
Thank u so much…
As it is very helpful & easy to learn. Thank u for the easy explanation…
Thanks, it is very easy
thank u so much .
Thank you. It was very helpful
Thanks mam