Question 1 Describe Bohr’s model of an atom?
Question 2 How did Neil Bohr’s explain the stability of atom?
Question 3 Why is an atom neutral inspite of the presence of charged particle in it?
Bohr’s Model Of An Atom
1) An atom is made up of three particles: electrons, protons and neutrons. Electrons have negative charge, protons have positive charge whereas neutrons have no charge. Due to the presence of equal number of negative electrons and positive protons, the atom on the whole is electrically neutral.
2) The protons and neutrons are located in a small nucleus at the centre of an atom. Due to the presence of protons, nucleus is positively charged.
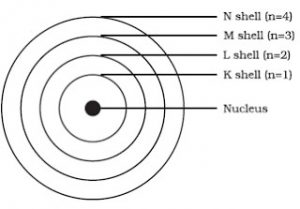
3) The electrons revolve around the nucleus in fixed circular paths called energy levels or shells. The energy levels or shells are represented in two ways:
a)K,L,M,N…….
b)1,2,3,4,5……
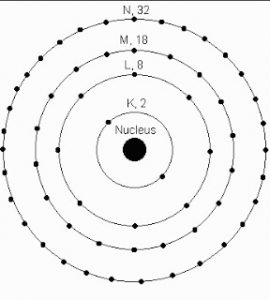
4) The first energy level or k shell can hold maximum of 2 electrons.
The second energy level or L shell can hold maximum of 8 electrons.
The third energy level or M shell can hold maximum of 18 electrons.
The fourth energy level or N shell can hold maximum of 32 electrons.
5) Each energy level or shell is associated with fixed amount of energy. There is no change in energy as long as they keep revolving in same energy level.
6) The change in energy of an electron take place when it
a) Jumps from lower energy level to higher energy level (gain energy)
b) Jumps from higher energy level to lower energy level (Loses energy)
Best
really nice context…. i love it!!!!!
Thanks
Osm
Thanks
it is the best description
Very good article and very helpful. Thank you!
Thanks for helping………
Thanks a lot.
NICE AND EASY LABELED DIAGRAM.
Thanks It’s easy to understand