Question 1 Define Evaporation ?
Question 2 What is the effect of temperature on rate of evaporation ?
Question 3 Why does our palm feel cold when we put some acetone or perfume in it?
Question 4 What type of clothes should we wear in summers and why.
Question 5 How does the water kept in an earthen pot become cold during summers.
Question 6 Why does a desert cooler cool better on a hot, dry day.
Question 7 How does sweating help keep our body cool on a hot day.
Question 8 Why does a liquid evaporates faster when kept in china dish.
Question 9 Name few factors that effect the rate of evaporation.
Question 10 Why people sprinkle water on ground during hot summer evening ?
Question 11 Why we are able to sip hot tea faster from a saucer than from a cup?
Contents
Evaporation
The process of liquid changing into vapours even below its boiling point is called as evaporation.
Some particles in a liquid always have more kinetic energy than the others. So, even when a liquid is well below its boiling point ,some of its particles have enough energy to break the forces of attraction between the particles and escape from the surface of the liquid in the form of vapours. Thus, the fast moving particles of a liquid are constantly escaping from the liquid to form vapour.
Factors affecting Evaporation
(1) Temperature
The rate of evaporation increases on increasing temperature of the liquid. When the temperature of a liquid is increased by heating it, more particles of the liquid get enough kinetic energy to go into vapour state. This increases the rate of evaporation.
(2) Surface Area
The rate of evaporation increases on increasing the surface area of the liquid.
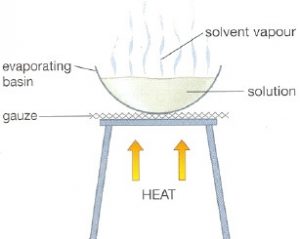
For Example: If the same liquid is kept in a test tube and in a china dish, then the liquid kept in the china dish evaporates more rapidly.
We spread out the washed clothes while drying to increase their surface area for rapid evaporation of water present in them.
(3) Humidity of Air
The amount of water vapours present in air is called humidity.
When the amount of water vapours present in the air is small it is called dry air.
When the amount of water vapours present in air is large it appears to be damp.
When the humidity of air is low, then the rate of evaporation is high and water evaporates more readily.
When the humidity of air is high, then the rate of evaporation is low and water evaporates very slowly.
(4) Wind Speed
The rate of evaporation of a liquid increases with the increasing wind speed.
When the speed of wind increases, the particles of water vapours move away with the wind, decreasing the amount of water vapours in the surrounding. This increases the rate of evaporation of water. The washed wet clothes dry more quickly on a windy day because evaporation is faster due to the high speed of the wind.
Cooling Caused by Evaporation
The cooling caused by evaporation is based on the fact when the liquid evaporates, it draws the latent heat of vaporisations from anything which it touches. By loosing heat this anything gets cooled.
(1) If we put a little of spirit at the back of our hand and wave it around, the spirit evaporates rapidly and our hand feels very cool. This is due to the fact that to change liquid to vapour state, spirit requires latent heat of vaporisation. The spirit takes this heat of vaporisation from our hand. The hand loses heat and gets cooled.
(2) During hot summer days, water is usually kept in an earthen pot to keep it cool. The earthen pot has a large number of extremely small pores in its walls. Some of the water continuously keeps seeping through these pores to the outside of the pot. This water evaporates continuously and takes the latent heat required for vaporisation from the earthen pot and remaining water. In this way, the remaining water loses heat and gets cooled.
(3) At many places especially in villages people often sprinkle water on the ground in front of their homes during summer evening. This water evaporates by taking latent heat of vaporisation from the ground and surrounding air. By losing heat, the place become cool and comfortable.
(4) Sweating is our body method of maintaining a constant temperature. On a hot day or after doing exercise, when our body temperature tends to rise too much, our sweat glands give out moisture on our skin. When this swat evaporates, it takes latent heat of vaporisation from our body.
(5) We should wear cotton clothes in hot summer days to keep cool and comfortable. We get lot of sweat on our body in hot summer days. Now cotton is a good absorber of water, so it absorbs the sweat from our body and expose it to the air for evaporation. The evaporation of this sweat cools our body.
(6) A desert cooler cools better on a hot and dry day because the higher temperature on a hot day increases the rate of evaporation of water and dryness of air also increases the rate of evaporation of water and due to increased rate of evaporation of water, a desert cooler cools better on a hot and dry day.
(7) We are able to sip hot tea faster from a saucer than from a cup. This is because saucer has large surface area. Due to the large surface area, the evaporation of hot tea from saucer is faster.
this is the first time ..i have understand clearly what is evaporation with real world applications .