Question 1 What are the characteristics of particle nature of matter ?
Question 2 What happens when a crystal of potassium permanganate is mixed with water ?
Question 3 Why there is no increase in volume when salt is dissolved in water ?
Question 4 How will you show that particles of matter are constantly moving ?
Question 5 A piece of chalk can be broken into small particles by hammering but a piece of iron cannot be broken into small particles by hammering. Why ?
Contents
Characteristics of Particle Nature of Matter
(1) The particles of matter are very very small in size
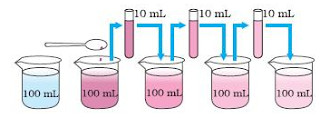
We take 2 to 3 crystals of potassium permanganate and dissolve in 100 ml of water in a beaker. We will get a deep purple coloured solution of potassium permanganate in water. Take 10 ml of deep purple solution of potassium permanganate from first beaker and mix it with 90ml of water present in second beaker, to dilute it. Due to this dilution, the colour of potassium permanganate solution from second beaker becomes a bit lighter. Now take 10 ml of potassium permanganate solution from the second beaker and mix it with 90ml of water present in the third beaker, to dilute further. The colour of solution becomes still lighter. Keep on diluting the potassium permanganate solution like this a number of times. In this way ,we get a very dilute solution of potassium permanganate in water but the water is still coloured. This experiment shows that just 2 -3 crystals of potassium permanganate can impart colour to large volume of water. Thus we conclude that each potassium permanganate crystal itself must be made up of millions of small particles.
(2) The particles of matter have spaces between them
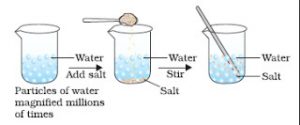
We take 100 ml of water in a beaker. Now add 50 g of salt to it. Dissolve the salt by stirring it with a glass rod. When all the salt has dissolved, we get a salt solution. We will find that the level of salt solution in the beaker is at the same mark where water level was initially in the beaker. When salt is dissolved in water, its crystals separate into very fine particles. These particles of salt go into the spaces between the various particles of water due to which there is no change in the volume of water on dissolving salt in it.
(3) The particles of matter are constantly moving
a) When we light or burn an incense stick in one corner of a room, its fragrance spreads in the whole room quickly. The particles move rapidly in all directions, mix with the moving particles of air in the room and reach every part of room quickly.
b) When few crystals of copper sulphate or potassium permanganate are placed in the beaker containing water, the whole beaker turns coloured. It is due to movement of both water and copper sulphate or potassium permanganate particles. Moving particles possesses kinetic energy. If the temperature increases, kinetic energy increases.
(4) Particles of matter attract each other
There are some forces of attraction between the particles of matter which bind them together. For Example : If we take a piece of chalk, a cube of ice or an iron nail and beat them with a hammer, we will find that it is very easy to break the piece of chalk into smaller particles, it requires more force to break a cube of ice, whereas iron nail does not break at all even with a large force. The forces of attraction between the particles of chalk is quite weak, the forces of attraction between the ice is a bit stronger whereas the forces of attraction between the particles of iron nail is very very strong.
Thank you so much