Question 1 Describe an activity to show that hydrochloric acid solution conduct electricity?
Question 2 Vinegar is a sour liquid. State whether vinegar conduct electricity or not?
Question 3 Describe an activity to show that sugar solution do not conduct electricity?
Question 4 How will you show that alcohol does not conduct electricity?
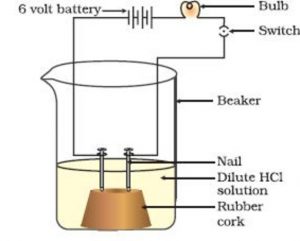
Take a small beaker. Fix two iron nails on a rubber cork about 1 cm apart and place this cork in the beaker.
The two iron nails will act as the two electrodes.
Connect the two nails to the two terminals of a battery by including a torch bulb and switch in the circuit.
Pour a solution of dilute hydrochloric acid in the beaker carefully. Now pass electric current through the hydrochloric acid solution by closing the switch.
We will observe that as soon as we switch on the current, the bulb starts glowing.
The bulb can glow only if the hydrochloric acid solution taken in the beaker conducts electricity. So the glowing of bulb this case tells us that hydrochloric acid solution conducts electricity.
Repeat this activity by taking sulphuric acid solution, sodium hydroxide solution, common salt solution, copper sulphate solution, vinegar and lemon juice in the beaker. The bulb glow again. This shows that all these solutions are conducting liquid.
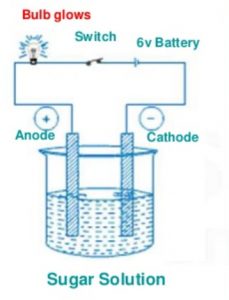
Let us now take sugar solution in the beaker and switch on the electric current by closing the switch.
We will observe that the bulb does not glow. This shows that sugar solution does not conduct electricity.
Sugar solution is not a conducting liquid. Repeat this activity by taking glucose solution, distilled water, alcohol solution, milk, vegetable oil and honey in the beaker. Bulb does not glow at all. They are not conducting liquids.
Leave a Reply