Question 1 Define the term chemical effects?
Question 2 What is electrolysis?
Question 3 What is an acidified water?
Question 4 Name one compound which is decomposed into hydrogen and oxygen by using chemical effects of electric current?
Question 5 What should be done to decompose water into hydrogen and oxygen?
Question 6 Acidified water is electrolysed by using carbon electrodes. What is produced at : Positive carbon electrode and negative carbon electrode?
Question 7 Explain the chemical effect of electric current with the help of an example?
Question 8 Explain why in the electrolysis of water, acidified water is used?
Question 9 State some of the characteristics of chemical changes brought about by the chemical effect of electric current?
Question 10 Name types of substances in which an electric current can produce a chemical effect?
Chemical Effects
Electric current can bring about chemical changes, so it said to have chemical effects.
When electric current is passed through acidified water by using carbon electrodes, then a chemical reaction takes place to form hydrogen gas and Oxygen gas.
A chemical compound water has been decomposed into two elements, hydrogen and oxygen, by the action of electric current.
It is actually a chemical decomposition reaction caused by passing an electric current through acidified.
The chemical decomposition produced by passing an electric current through a conducting liquid is called electrolysis. The water containing a little of acid is called as acidified water.
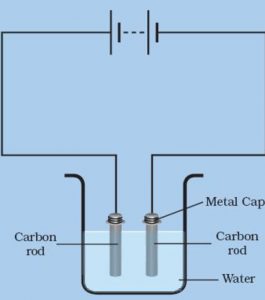
Activity : To demonstrate the chemical effect of electric current.
Take out two carbon rods from discarded dry cells carefully. Clean their metal caps with Sand paper. Wrap copper wires around the metal caps of the carbon rods and join them to a battery through a switch. Take 250 ml of a water in a beaker. Add a few drops of dilute sulphuric acid to water to make it more conducting. Now immerse carbon rods in acidified water in the beaker. Make sure that the metal cap of the carbon rods are above the level of water in the beaker. Pass electric current through acidified water in the beaker by closing the switch. Wait for 4 to 5 minutes and observe the two carbon electrodes carefully. The bubbles of gases are produced at the two carbon electrodes. The formation of gas bubbles at the two carbon electrodes shows that a chemical change has taken place in water on passing electric current through it.
If electric current is passed through acidified water, then bubbles of Oxygen gas and hydrogen gas are produced at the two electrodes in immersed in it.
(1) Oxygen gas is formed at the positive electron that is anode which is connected to the positive terminal of the battery.
(2) Hydrogen gas is formed at the negative electrode that is cathode which is connected to the negative terminal of the battery.
An electric current can also produce a chemical effect on some other substances such as acids, bases, salts and certain molten compounds. When an electric currents is passed through these liquids chemical reaction takes place. When an electric current flows through a conducting solution, it causes a chemical reaction or chemical change.
The chemical reaction brought about by an electric current may produce the following effects:
(1) Bubbles of a gas may be formed on electrodes.
(2) Deposits of metals may form an electrodes
(3) Changes in colour of solution may occur.
The chemical effects produced by an electric current depends on the nature of conducting solution, and on the nature of electrodes used for passing the electric current.
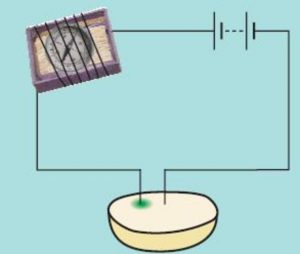
Activity :Demonstrate the change in colour caused by the chemical effects of electric current
Cut a potato into two halves. Take one piece of cut potato and insert two Iron nails into it a little distance apart from one another. The iron nails are the two electrodes. Connect the two terminals of a battery to the to Iron nails by including a compass and a switch in the circuit. Pass the electric current through cut potato piece by closing the switch. We will observe a deflection in the compass needle showing that potato conduct electricity to some extent .Let us continue to pass electric current through potato piece for about half an hour. We will observe a greenish blue spot on the cut surface of potato around the iron nail which is connected to the negative terminal of the battery. The formation of a greenish blue spot around the positive electrode inserted in the surface of a cut potato shows that the chemical effect of current can bring about change in the colour of a conducting solution.
Leave a Reply