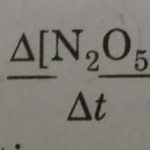Molecularity of a Reaction For a chemical reaction to occur, the reacting molecules must collide with each other. The number of reacting species (molecules, atoms or ions) which collide simultaneously to bring about a chemical reaction is called molecularity of a reaction. If a reaction involves the decomposition of only a single species, the molecularity is one or it is … [Read more...] about Molecularity of a Reaction
Class 12
Order of a Reaction
Order of a Reaction The order of a reaction is defined as: the sum of the powers to which the concentration terms are raised in the rate law equation to express the observed rate of the reaction. The power of the concentration of a particular reactant in the rate law is called the order of the reaction with respect to that reactant. If the rate of a reaction, aA + bB … [Read more...] about Order of a Reaction
Factors Influencing Rates of Chemical Reactions
Factors Influencing Rates of Chemical Reaction There are a number of factors which influence the rate of a reaction. Some of the important factors are: 1) Concentration of the reacting species. 2) Temperature of the system 3) Nature of the reactants and products. 4) Presence of a catalyst. 5) Surface area. 6) Exposure to radiation. 1) Concentration of the … [Read more...] about Factors Influencing Rates of Chemical Reactions
Rate of a Chemical Reaction
The feasibility of a reaction under the given experimental conditions can be predicted on, the basis of decrease in Gibbs energy (ΔG < 0). The extent to which a reaction proceeds can be explained with the help of equilibrium constant of the reaction. The branch of chemistry which deals with the rates of chemical reactions and the factors which influence the rates of … [Read more...] about Rate of a Chemical Reaction
Corrosion
Corrosion When metals are exposed to atmospheric conditions, they react with air or water in the environment to form undesirable compounds (usually oxides). This process is called corrosion. The least active metals such as gold, platinum and palladium are attacked by environment. For example: Silver tarnishes, copper develops a green coating, lead or stainless steel lose … [Read more...] about Corrosion