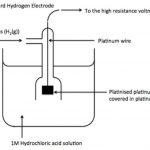
Electrode Potential The flow of electric current in an electrochemical cell indicates that a potential difference exists between two electrodes. When an electrode say copper, is immersed in a solution of its ions, then either of the following three possibilities can take place: a) The metal ions (Cu2+) may collide with the electrode and do not undergo any change. b) … [Read more...] about Electrode Potential and E.M.F. of a Galvanic Cell
Chemistry
Electrochemical Cells
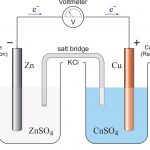
Electrochemical Cells The chemical changes which involve the flow of electric current are called electrochemical changes. These are broadly of two types: 1) Electrochemical cells or Galvanic cells These constitute the electrochemical reactions in which chemical energy is converted to electrical energy. In these cells, spontaneous redox reaction is used to generate an … [Read more...] about Electrochemical Cells
Variation of Conductivity and Molar Conductivity
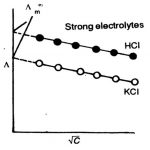
Variation of Conductivity and Molar conductivity with conductivity Electrolytic conductance decreases with increase in concentration or increases with increase in dilution. This is because conductance of ions is due to the presence of ions in the solution. The greater the number of ions, the greater is the conductance. As with dilution, more ions are produced in solution so … [Read more...] about Variation of Conductivity and Molar Conductivity
Electrolytic Conduction
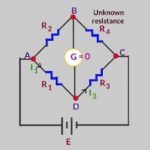
Electrolytic Conduction When a voltage is applied to the electrodes dipped into an electrolytic solution, ions of the electrolyte move and, therefore, electric current flows through the electrolytic solution. The power of the electrolytes to conduct electric current is termed conductance or conductivity. Electrolytic solutions also obey Ohm's law. Ohm's Law This law … [Read more...] about Electrolytic Conduction
Classification of Electrolytes
Electrochemistry The branch of science which deals with the production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non- spontaneous chemical transformations is called electrochemistry. Redox Reaction Oxidation is a process which involves loss of electrons and reduction is a process which involves … [Read more...] about Classification of Electrolytes